| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:21:17 UTC |
|---|
| Update Date | 2016-11-09 01:17:47 UTC |
|---|
| Accession Number | CHEM023893 |
|---|
| Identification |
|---|
| Common Name | ent-Epiafzelechin-(2alpha->7,4alpha->8)-catechin |
|---|
| Class | Small Molecule |
|---|
| Description | Ent-epiafzelechin-(2alpha->7,4alpha->8)-catechin is a member of the class of compounds known as biflavonoids and polyflavonoids. Biflavonoids and polyflavonoids are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. Ent-epiafzelechin-(2alpha->7,4alpha->8)-catechin is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Ent-epiafzelechin-(2alpha->7,4alpha->8)-catechin can be found in peach, which makes ent-epiafzelechin-(2alpha->7,4alpha->8)-catechin a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
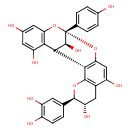
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| ent-Epiafzelechin-(2a->7,4a->8)-catechin | Generator | | ent-Epiafzelechin-(2α->7,4α->8)-catechin | Generator |
|
|---|
| Chemical Formula | C30H24O11 |
|---|
| Average Molecular Mass | 560.505 g/mol |
|---|
| Monoisotopic Mass | 560.132 g/mol |
|---|
| CAS Registry Number | 135820-74-5 |
|---|
| IUPAC Name | (5R,6S,13R,21S)-5-(3,4-dihydroxyphenyl)-13-(4-hydroxyphenyl)-4,12,14-trioxapentacyclo[11.7.1.0²,¹¹.0³,⁸.0¹⁵,²⁰]henicosa-2(11),3(8),9,15(20),16,18-hexaene-6,9,17,19,21-pentol |
|---|
| Traditional Name | (5R,6S,13R,21S)-5-(3,4-dihydroxyphenyl)-13-(4-hydroxyphenyl)-4,12,14-trioxapentacyclo[11.7.1.0²,¹¹.0³,⁸.0¹⁵,²⁰]henicosa-2(11),3(8),9,15(20),16,18-hexaene-6,9,17,19,21-pentol |
|---|
| SMILES | O[C@H]1CC2=C(O[C@@H]1C1=CC(O)=C(O)C=C1)C1=C(O[C@]3(OC4=C(C1[C@@H]3O)C(O)=CC(O)=C4)C1=CC=C(O)C=C1)C=C2O |
|---|
| InChI Identifier | InChI=1S/C30H24O11/c31-14-4-2-13(3-5-14)30-29(38)26(24-20(36)8-15(32)9-22(24)40-30)25-23(41-30)11-18(34)16-10-21(37)27(39-28(16)25)12-1-6-17(33)19(35)7-12/h1-9,11,21,26-27,29,31-38H,10H2/t21-,26?,27+,29-,30+/m0/s1 |
|---|
| InChI Key | MXKKFADFYXJREN-YBYURAQPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biflavonoids and polyflavonoids. These are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Biflavonoids and polyflavonoids |
|---|
| Direct Parent | Biflavonoids and polyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - A-type proanthocyanidin
- Bi- and polyflavonoid skeleton
- Proanthocyanidin
- Catechin
- Pyranoflavonoid
- Flavan-3-ol
- Hydroxyflavonoid
- 3'-hydroxyflavonoid
- 7-hydroxyflavonoid
- 5-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 3-hydroxyflavonoid
- Flavan
- Pyranochromene
- Chromane
- 1-benzopyran
- Benzopyran
- Catechol
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Ketal
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Ether
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-1113390000-8e4c1dde10b9d9acb951 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-1424940000-4132471214d329c47c74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9412010000-398275f8f0e6dc21b904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0110190000-44901bbf8647b9f7db75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pbc-1921360000-14bb79c5fc2a2c9b0229 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05di-0920000000-3def5e5ebd4ab57aa9b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000090000-e26044261e1753b3f2e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000590000-e8e38a0336330046a7ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-0410950000-7c5781f1439040ae4bc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-f1af39007edb9d799840 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000390000-16c24486204f5dfa0c33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05i0-1351930000-f8e812ceaea62348fa83 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB001643 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|