| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:21:10 UTC |
|---|
| Update Date | 2016-11-09 01:17:47 UTC |
|---|
| Accession Number | CHEM023889 |
|---|
| Identification |
|---|
| Common Name | Epicatechin-(4beta->8)-catechin 3-gallate |
|---|
| Class | Small Molecule |
|---|
| Description | A gallate ester obtained by formal condensation of the carboxy group of gallic acid with the (3R)-hydroxy group of procyanidin B1. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
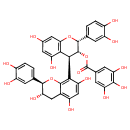
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Epicatechin-(4beta->8)-(+)-catechin-3-O-gallate | ChEBI | | (-)-Epicatechin-(4b->8)-(+)-catechin-3-O-gallate | Generator | | (-)-Epicatechin-(4b->8)-(+)-catechin-3-O-gallic acid | Generator | | (-)-Epicatechin-(4beta->8)-(+)-catechin-3-O-gallic acid | Generator | | (-)-Epicatechin-(4β->8)-(+)-catechin-3-O-gallate | Generator | | (-)-Epicatechin-(4β->8)-(+)-catechin-3-O-gallic acid | Generator | | Procyanidin b1 3-O-gallic acid | Generator | | Epicatechin-(4b->8)-catechin 3-gallate | Generator | | Epicatechin-(4b->8)-catechin 3-gallic acid | Generator | | Epicatechin-(4beta->8)-catechin 3-gallic acid | Generator | | Epicatechin-(4β->8)-catechin 3-gallate | Generator | | Epicatechin-(4β->8)-catechin 3-gallic acid | Generator | | Procyanidin dimer b1 3-gallic acid | Generator |
|
|---|
| Chemical Formula | C37H30O16 |
|---|
| Average Molecular Mass | 730.625 g/mol |
|---|
| Monoisotopic Mass | 730.153 g/mol |
|---|
| CAS Registry Number | 79907-45-2 |
|---|
| IUPAC Name | (2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-8-yl]-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | (2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-8-yl]-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | O[C@H]1CC2=C(O[C@@H]1C1=CC(O)=C(O)C=C1)C([C@@H]1[C@@H](OC(=O)C3=CC(O)=C(O)C(O)=C3)[C@H](OC3=CC(O)=CC(O)=C13)C1=CC(O)=C(O)C=C1)=C(O)C=C2O |
|---|
| InChI Identifier | InChI=1S/C37H30O16/c38-16-9-23(44)29-28(10-16)51-34(14-2-4-19(40)22(43)6-14)36(53-37(50)15-7-25(46)32(49)26(47)8-15)31(29)30-24(45)12-20(41)17-11-27(48)33(52-35(17)30)13-1-3-18(39)21(42)5-13/h1-10,12,27,31,33-34,36,38-49H,11H2/t27-,31+,33+,34+,36+/m0/s1 |
|---|
| InChI Key | BXWABJPTCUDBMM-NNJCKQBNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biflavonoids and polyflavonoids. These are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Biflavonoids and polyflavonoids |
|---|
| Direct Parent | Biflavonoids and polyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - B-type proanthocyanidin
- Bi- and polyflavonoid skeleton
- Proanthocyanidin
- Catechin gallate
- Catechin
- 3'-hydroxyflavonoid
- 3-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Flavan-3-ol
- Hydroxyflavonoid
- Flavan
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- 1-benzopyran
- Benzopyran
- Chromane
- Benzoate ester
- Pyrogallol derivative
- Benzoic acid or derivatives
- Benzenetriol
- Benzoyl
- Catechol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Carboxylic acid ester
- Secondary alcohol
- Ether
- Oxacycle
- Polyol
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0in9-0510390800-feb08379d62d393c9bf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0963822100-eff2eaeabd1899fd608d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-0972010000-dd71d19c0af6655f5ffb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0400031900-724cb0043d0c65a45983 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fbi-0920022100-ad8151479004ac1e19d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-0911000000-735a8e11f1df71bb3bbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000900-87b504be1441dfed220d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-0000015900-085f212c140946518cd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0400039200-b701176c75656d251133 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0100092600-3f1c5cfe85f3bfc2b16d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-0150389800-b41848ef9bc79a41712e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fk9-0600129300-487b7c7ff5786cd1de95 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0301931 |
|---|
| FooDB ID | FDB001639 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10290148 |
|---|
| ChEBI ID | 75651 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|