| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:20:43 UTC |
|---|
| Update Date | 2016-11-09 01:17:47 UTC |
|---|
| Accession Number | CHEM023876 |
|---|
| Identification |
|---|
| Common Name | 4-Metyl-5-(beta-hydroxyethyl)thiazole phosphate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
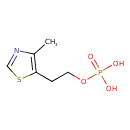
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Methyl-5-(2-phosphoethyl)-thiazole | ChEBI | | 4-Methyl-5-(2-phosphono-oxyethyl)-thiazole | ChEBI | | 4-Methyl-5-(2-phosphonooxyethyl)-thiazole | ChEBI | | PHOSPHORIC ACID mono-[2-(4-methyl-thiazol-5-yl)-ethyl] ester | ChEBI | | 4-Methyl-5-(2-phosphonooxyethyl)thiazole | Kegg | | 5-(2-Hydroxyethyl)-4-methylthiazole phosphate | Kegg | | HET-p | Kegg | | PHOSPHate mono-[2-(4-methyl-thiazol-5-yl)-ethyl] ester | Generator | | 5-(2-Hydroxyethyl)-4-methylthiazole phosphoric acid | Generator | | 4-Methyl-5-hydroxyethylthiazole phosphoric acid | Generator | | 4-METHYL-5-hydroxyethylthiazole phosphATE | ChEBI | | 4-Metyl-5-(b-hydroxyethyl)thiazole phosphate | Generator | | 4-Metyl-5-(b-hydroxyethyl)thiazole phosphoric acid | Generator | | 4-Metyl-5-(beta-hydroxyethyl)thiazole phosphoric acid | Generator | | 4-Metyl-5-(β-hydroxyethyl)thiazole phosphate | Generator | | 4-Metyl-5-(β-hydroxyethyl)thiazole phosphoric acid | Generator |
|
|---|
| Chemical Formula | C6H10NO4PS |
|---|
| Average Molecular Mass | 223.187 g/mol |
|---|
| Monoisotopic Mass | 223.007 g/mol |
|---|
| CAS Registry Number | 3269-79-2 |
|---|
| IUPAC Name | [2-(4-methyl-1,3-thiazol-5-yl)ethoxy]phosphonic acid |
|---|
| Traditional Name | 2-(4-methyl-1,3-thiazol-5-yl)ethoxyphosphonic acid |
|---|
| SMILES | CC1=C(CCOP(O)(O)=O)SC=N1 |
|---|
| InChI Identifier | InChI=1S/C6H10NO4PS/c1-5-6(13-4-7-5)2-3-11-12(8,9)10/h4H,2-3H2,1H3,(H2,8,9,10) |
|---|
| InChI Key | OCYMERZCMYJQQO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4,5-disubstituted thiazoles. 4,5-disubstituted thiazoles are compounds containing a thiazole ring substituted at positions 4 and 5 only. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Thiazoles |
|---|
| Direct Parent | 4,5-disubstituted thiazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monoalkyl phosphate
- 4,5-disubstituted 1,3-thiazole
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9300000000-4b265fbb33f5d2a284a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-1970000000-6d53ae2ae5cf22f5c35d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1900000000-2a7dfc383c0b8f0bc737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-8900000000-f80f35678c9e0a53bfdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-9170000000-0f15ea5b2875cc1966c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-9be355f278967310c343 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-3af10cdc8386468900f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-0690000000-c5a85288f3c0ae83b359 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-4900000000-5a3ba3a8afe5cee644b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03gi-9600000000-47caa828201211845c2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fs-9050000000-392915fcb08678ef8065 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-a5e502a2627af2048a1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a5e502a2627af2048a1f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB03145 |
|---|
| HMDB ID | HMDB0304185 |
|---|
| FooDB ID | FDB030511 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00007608 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 1105 |
|---|
| ChEBI ID | 17857 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C04327 |
|---|
| YMDB ID | YMDB00319 |
|---|
| ECMDB ID | ECMDB23089 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|