| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:20:08 UTC |
|---|
| Update Date | 2016-11-09 01:17:47 UTC |
|---|
| Accession Number | CHEM023860 |
|---|
| Identification |
|---|
| Common Name | Xanthoangelol B |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
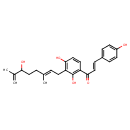
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C25H28O5 |
|---|
| Average Molecular Mass | 408.487 g/mol |
|---|
| Monoisotopic Mass | 408.194 g/mol |
|---|
| CAS Registry Number | 132998-81-3 |
|---|
| IUPAC Name | (2E)-1-{2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethylocta-2,7-dien-1-yl]phenyl}-3-(4-hydroxyphenyl)prop-2-en-1-one |
|---|
| Traditional Name | xanthoangelol B |
|---|
| SMILES | CC(=C)C(O)CC\C(C)=C\CC1=C(O)C=CC(C(=O)\C=C\C2=CC=C(O)C=C2)=C1O |
|---|
| InChI Identifier | InChI=1S/C25H28O5/c1-16(2)22(27)13-5-17(3)4-11-20-24(29)15-12-21(25(20)30)23(28)14-8-18-6-9-19(26)10-7-18/h4,6-10,12,14-15,22,26-27,29-30H,1,5,11,13H2,2-3H3/b14-8+,17-4+ |
|---|
| InChI Key | NCHZAFAGBAEJJJ-BAYITLGHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-prenylated chalcones. These are chalcones featuring a C5-isoprenoid unit at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | 3-prenylated chalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-prenylated chalcone
- 2'-hydroxychalcone
- Cinnamylphenol
- Hydroxycinnamic acid or derivatives
- Fatty alcohol
- Benzoyl
- Resorcinol
- Styrene
- Aryl ketone
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Fatty acyl
- Monocyclic benzene moiety
- Vinylogous acid
- Alpha,beta-unsaturated ketone
- Acryloyl-group
- Enone
- Secondary alcohol
- Ketone
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0339500000-9327b2eb8e2f7c38aafe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05te-4943000000-ec00e56bd5b81e34a95b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xs-6910000000-8804ec65de519be5ed17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0122900000-3a5bed33452f82cf4095 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-0759700000-7fec078d8c566873edbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-5693000000-f276f147c171fa9f4117 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0109100000-816212a0c72466d31a30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-1719000000-3b05df514076581d9194 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aba-0922000000-bfe130b4a57716e5accd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-0009600000-9bd6f8c3066a0e45a778 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0avr-1329200000-773debca87c1fd597c43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2923000000-b43d72f251967919b7b2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0301910 |
|---|
| FooDB ID | FDB001602 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8584617 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|