| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:11:20 UTC |
|---|
| Update Date | 2016-11-09 01:17:44 UTC |
|---|
| Accession Number | CHEM023621 |
|---|
| Identification |
|---|
| Common Name | Austalide B |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
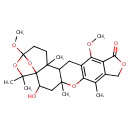
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H34O8 |
|---|
| Average Molecular Mass | 474.543 g/mol |
|---|
| Monoisotopic Mass | 474.225 g/mol |
|---|
| CAS Registry Number | 81543-02-4 |
|---|
| IUPAC Name | 2-hydroxy-13,20-dimethoxy-4,7,17,22,22-pentamethyl-5,10,21,23-tetraoxahexacyclo[18.2.1.0¹,¹⁷.0⁴,¹⁶.0⁶,¹⁴.0⁸,¹²]tricosa-6(14),7,12-trien-11-one |
|---|
| Traditional Name | 2-hydroxy-13,20-dimethoxy-4,7,17,22,22-pentamethyl-5,10,21,23-tetraoxahexacyclo[18.2.1.0¹,¹⁷.0⁴,¹⁶.0⁶,¹⁴.0⁸,¹²]tricosa-6(14),7,12-trien-11-one |
|---|
| SMILES | COC1=C2C(=O)OCC2=C(C)C2=C1CC1C(C)(CC(O)C34OC(CCC13C)(OC)OC4(C)C)O2 |
|---|
| InChI Identifier | InChI=1S/C26H34O8/c1-13-15-12-31-21(28)18(15)20(29-6)14-10-16-23(4)8-9-25(30-7)33-22(2,3)26(23,34-25)17(27)11-24(16,5)32-19(13)14/h16-17,27H,8-12H2,1-7H3 |
|---|
| InChI Key | ZVFMDVFPBVFGPG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthenes. These are polycyclic aromatic compounds containing a xanthene moiety, which consists of two benzene rings joined to each other by a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthene
- Phthalide
- Isobenzofuranone
- Isocoumaran
- Anisole
- Alkyl aryl ether
- Carboxylic acid orthoester
- Ortho ester
- Oxepane
- Benzenoid
- Oxane
- Meta-dioxolane
- Cyclic alcohol
- Secondary alcohol
- Orthocarboxylic acid derivative
- Carboxylic acid ester
- Lactone
- Oxacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Ether
- Organic oxide
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-3440900000-487775a98907abeed7f4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-001i-6441390000-8efa1635aa8e2255887e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0080900000-8a49d194b1ae5a0ec940 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0190500000-ff91bc80465e1ac2297f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9770100000-30559334e8d203ca565a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-e4ef1b4dd6d68ab3d652 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0596-0110900000-66d5190c99bf481b5387 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-0928600000-6de32434422a13fb8be6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-1a23a00ec07d277e3624 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1000900000-bc7a855737e26d9598f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-074i-1205900000-6b45e1dda1f1ccaed33a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000900000-97482fd0ad867393fca3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003s-0110900000-1441415e2a98ebb41aa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07ou-1020900000-badb71513ebe7b107501 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054593 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 138997 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 157978 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|