| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:06:36 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023512 |
|---|
| Identification |
|---|
| Common Name | L-Sorbose |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
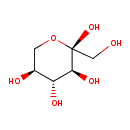
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-L-Sorbopyranose | HMDB | | Hex-2-ulose | HMDB | | L(-) Sorbose for biochemistry | HMDB | | L(-)-Sorbose | HMDB | | L-(-)-Sorbose | HMDB | | L-(-)-Sorbose 99% | HMDB | | L-1,3,4,5,6-Pentahydroxyhexan-2-one | HMDB | | L-Sorbinose | HMDB | | L-Xylo-2-hexulose | HMDB | | L-Xylo-hexulose | HMDB | | Sorbin | HMDB | | Sorbinose | HMDB | | Sorbose | HMDB | | Xylo-hexulose | HMDB | | alpha-L-Xylo-hexopyranos-2-ulose | HMDB | | Α-L-xylo-hexopyranos-2-ulose | HMDB | | Α-L-sorbopyranose | HMDB | | a-L-Sorbopyranose | HMDB |
|
|---|
| Chemical Formula | C18H36O18 |
|---|
| Average Molecular Mass | 540.468 g/mol |
|---|
| Monoisotopic Mass | 540.190 g/mol |
|---|
| CAS Registry Number | 470-15-5 |
|---|
| IUPAC Name | (2R,3S,4R,5S)-2-(hydroxymethyl)oxane-2,3,4,5-tetrol |
|---|
| Traditional Name | α-L-sorbopyranose |
|---|
| SMILES | OCC(O)C(O)C(O)C(=O)CO.OCC1OC(O)(CO)C(O)C1O.OCC1(O)OCC(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/3C6H12O6/c7-2-6(11)5(10)4(9)3(8)1-12-6;7-1-3-4(9)5(10)6(11,2-8)12-3;7-1-3(9)5(11)6(12)4(10)2-8/h2*3-5,7-11H,1-2H2;3,5-9,11-12H,1-2H2 |
|---|
| InChI Key | JCOXAIPZGMCTBN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monosaccharides. Monosaccharides are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Monosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxane
- Monosaccharide
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08fr-9700000000-3e5e0a3aa6c676c97701 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-004i-8422790000-fda815da7846441c6ea7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-0900000000-ad56c2283c4948268668 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00ri-9600000000-f53f9ae5db12b8c07611 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0079-9200000000-c3dabd4edd0fd062cfd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-014681a0ceccca243630 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-2900000000-fdd4f1c2b01b7cd4a621 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0c30-9200000000-a3afc1e71049f23145b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fu-9700000000-86b1b9f75df959e2158f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-024i-9800000000-1acd95dd1e6f8744308f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-545f59c3707e4298b57c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01pk-0900000000-d9de28ed437f1079125e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-9700000000-21b6c695fb9bdd5ae08d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-7c49e3a2d60cfed68a76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-6900000000-def874fe6470032fdd27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-9100000000-b495c0f16251b5ee98e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-d526231c78d5ea2b9be5 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001266 |
|---|
| FooDB ID | FDB001126 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019630 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | SORBOSE |
|---|
| METLIN ID | 6121 |
|---|
| PDB ID | SOE |
|---|
| Wikipedia Link | Sorbose |
|---|
| Chemspider ID | 390208 |
|---|
| ChEBI ID | 10295 |
|---|
| PubChem Compound ID | 441484 |
|---|
| Kegg Compound ID | C08356 |
|---|
| YMDB ID | YMDB00204 |
|---|
| ECMDB ID | ECMDB01266 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Banjopnoppakun, Tanaphat; Moonmangmee, Somporn; Moonmangmee, Duangtip. Production of L-sorbose by thermotolerant acetic acid bacteria. Proceeding of the Kasetsart University Annual Conference, 44th, Bangkok, Thailand, Jan. 30-Feb. 2, 2006 (2006), 117-123. | | 2. Banjopnoppakun, Tanaphat; Moonmangmee, Somporn; Moonmangmee, Duangtip. Production of L-sorbose by thermotolerant acetic acid bacteria. Proceeding of the Kasetsart University Annual Conference, 44th, Bangkok, Thailand, Jan. 30-Feb. 2, 2006 (2006), 117-123. | | 3. Wang C, So SY, Wong KK, So WW, Chan SY: Chronic sinopulmonary disease in Chinese patients with obstructive azoospermia. J Androl. 1987 Jul-Aug;8(4):225-9. | | 4. Gryz EA, Galicka-Latala D, Szczudlik A, Sieradzki J: [Etiopathogenesis of diabetic neuropathy]. Przegl Lek. 2000;57(12):727-31. | | 5. Kossi J, Peltonen J, Uotila P, Laato M: Differential effects of hexoses and sucrose, and platelet-derived growth factor isoforms on cyclooxygenase-1 and -2 mRNA expression in keloid, hypertrophic scar and granulation tissue fibroblasts. Arch Dermatol Res. 2001 Mar;293(3):126-32. | | 6. Gross KC, Houghton MP, Senterfit LB: Presumptive speciation of Streptococcus bovis and other group D streptococci from human sources by using arginine and pyruvate tests. J Clin Microbiol. 1975 Jan;1(1):54-60. | | 7. Fukasawa M, Takayama E, Shinomiya N, Okumura A, Rokutanda M, Yamamoto N, Sakakibara R: Identification of the promoter region of human placental 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Biochem Biophys Res Commun. 2000 Jan 27;267(3):703-8. | | 8. Le KA, Tappy L: Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006 Jul;9(4):469-75. | | 9. Fukasawa M, Tsuchiya T, Takayama E, Shinomiya N, Uyeda K, Sakakibara R, Seki S: Identification and characterization of the hypoxia-responsive element of the human placental 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. J Biochem. 2004 Sep;136(3):273-7. | | 10. Yanez AJ, Bertinat R, Spichiger C, Carcamo JG, de Los Angeles Garcia M, Concha II, Nualart F, Slebe JC: Novel expression of liver FBPase in Langerhans islets of human and rat pancreas. J Cell Physiol. 2005 Oct;205(1):19-24. | | 11. Lee YH, Li Y, Uyeda K, Hasemann CA: Tissue-specific structure/function differentiation of the liver isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 2003 Jan 3;278(1):523-30. Epub 2002 Oct 11. | | 12. Minchenko O, Opentanova I, Minchenko D, Ogura T, Esumi H: Hypoxia induces transcription of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-4 gene via hypoxia-inducible factor-1alpha activation. FEBS Lett. 2004 Oct 8;576(1-2):14-20. | | 13. Andrade-Rocha FT: Physical analysis of ejaculate to evaluate the secretory activity of the seminal vesicles and prostate. Clin Chem Lab Med. 2005;43(11):1203-10. | | 14. Roy S, Banerjee A, Pandey HC, Singh G, Kumari GL: Application of seminal germ cell morphology and semen biochemistry in the diagnosis and management of azoospermic subjects. Asian J Androl. 2001 Mar;3(1):55-62. | | 15. Nakamura J, Koh N, Sakakibara F, Hamada Y, Wakao T, Sasaki H, Mori K, Nakashima E, Naruse K, Hotta N: Diabetic neuropathy in sucrose-fed Otsuka Long-Evans Tokushima fatty rats: effect of an aldose reductase inhibitor, TAT. Life Sci. 1997;60(21):1847-57. | | 16. Blakemore SJ, Aledo JC, James J, Campbell FC, Lucocq JM, Hundal HS: The GLUT5 hexose transporter is also localized to the basolateral membrane of the human jejunum. Biochem J. 1995 Jul 1;309 ( Pt 1):7-12. | | 17. Abou El Fadil-Nicol F, Berger F, Descroix-Vagne M, Pansu D: Presence of sorbin in human digestive tract and endocrine digestive tumours. Gut. 2000 Feb;46(2):182-90. | | 18. Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L: Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005 Jul;54(7):1907-13. | | 19. Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R: High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002 Oct 15;62(20):5881-7. | | 20. Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT: Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr. 2004 Apr;43(2):116-24. Epub 2004 Jan 6. | | 21. Ludwig M, Vidal A, Diemer T, Pabst W, Failing K, Weidner W: Seminal secretory capacity of the male accessory sex glands in chronic pelvic pain syndrome (CPPS)/chronic prostatitis with special focus on the new prostatitis classification. Eur Urol. 2002 Jul;42(1):24-8. | | 22. Massucco P, Mattiello L, Russo I, Traversa M, Doronzo G, Anfossi G, Trovati M: High glucose rapidly activates the nitric oxide/cyclic nucleotide pathway in human platelets via an osmotic mechanism. Thromb Haemost. 2005 Mar;93(3):517-26. | | 23. Sugisawa T, Miyazaki T, Hoshino T: Microbial production of L-ascorbic acid from D-sorbitol, L-sorbose, L-gulose, and L-sorbosone by Ketogulonicigenium vulgare DSM 4025. Biosci Biotechnol Biochem. 2005 Mar;69(3):659-62. doi: 10.1271/bbb.69.659. |
|
|---|