| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:06:13 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023503 |
|---|
| Identification |
|---|
| Common Name | Paucine |
|---|
| Class | Small Molecule |
|---|
| Description | Paucine is found in avocado. Paucine is an alkaloid from the famine food Pentaclethra macrophylla and from Persea gratissima (avocado |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
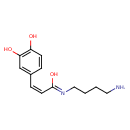
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E)-N-(4-Aminobutyl)-3-(3,4-dihydroxyphenyl)acrylamide | HMDB | | (2E)-N-(4-Aminobutyl)-3-(3,4-dihydroxyphenyl)prop-2-enamide | HMDB | | Caffeoylputrescine | HMDB | | N-(3,4-Dihydroxycinnamoyl)-1,4-butanediamine | HMDB | | N-(4-Aminobutyl)-3,4-dihydroxy-(e)-cinnamamide | HMDB | | N-(4-Aminobutyl)-3-(3,4-dihydroxyphenyl)-2-propanamide, 9ci | HMDB | | N-Caffeoylputrescine | HMDB | | (2Z)-N-(4-Aminobutyl)-3-(3,4-dihydroxyphenyl)prop-2-enimidate | Generator |
|
|---|
| Chemical Formula | C13H18N2O3 |
|---|
| Average Molecular Mass | 250.294 g/mol |
|---|
| Monoisotopic Mass | 250.132 g/mol |
|---|
| CAS Registry Number | 29554-26-5 |
|---|
| IUPAC Name | (Z,2Z)-N-(4-aminobutyl)-3-(3,4-dihydroxyphenyl)propa-2-enimidic acid |
|---|
| Traditional Name | (Z,2Z)-N-(4-aminobutyl)-3-(3,4-dihydroxyphenyl)propa-2-enimidic acid |
|---|
| SMILES | NCCCC\N=C(/O)\C=C/C1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C13H18N2O3/c14-7-1-2-8-15-13(18)6-4-10-3-5-11(16)12(17)9-10/h3-6,9,16-17H,1-2,7-8,14H2,(H,15,18)/b6-4- |
|---|
| InChI Key | KTZNZCYTXQYEHT-XQRVVYSFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenediols |
|---|
| Direct Parent | Catechols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Catechol
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Carboximidic acid
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q9-9710000000-83e750853026126b3eae | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0ue9-7006900000-fde5744144b4802e0811 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-9160000000-ca008be3a6368fda476b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-9100000000-fe38c7990c4a88bc0993 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9100000000-34629f1c0162d7f84985 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1290000000-6e558b4b21a5ac0393a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01rb-5960000000-ea3a5da9464b5421a436 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-99fa8fe91e106b64249c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-ff1daeeaa5b95133695a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1590000000-dde974faae7120ad783c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-4900000000-3d14b43a0d7e93a7a6e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000t-0290000000-f73636a887a20e978dbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1910000000-35af733e55fca776832c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0019-3900000000-8f1473c177e4fd4fa3b1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029876 |
|---|
| FooDB ID | FDB001105 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002719 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776796 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131750921 |
|---|
| Kegg Compound ID | C03002 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|