| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:06:03 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023499 |
|---|
| Identification |
|---|
| Common Name | gamma-Asarone |
|---|
| Class | Small Molecule |
|---|
| Description | gamma-Asarone is found in herbs and spices. gamma-Asarone is a constituent of Acorus calamus (sweet flag) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
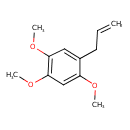
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-Asarone | Generator | | Γ-asarone | Generator | | 1,2,4-Trimethoxy-5-(2-propenyl)-benzene | HMDB | | 1-Allyl-2,4,5-trimethoxybenzene | HMDB | | 2,4,5-Trimethoxyallylbenzene | HMDB | | Euasarone | HMDB | | Isoasarone | HMDB | | Sekishon | HMDB | | (e)-Asarone | HMDB | | 2,4,5-Trimethoxypropen-1-ylbenzene | HMDB | | alpha-Asarone | HMDB | | Asarone | HMDB | | beta-Asarone | HMDB | | cis-beta-Asarone | HMDB | | Asarone, (e)-isomer | HMDB | | cis-Isoelemicin | HMDB | | (Z)-Asarone | HMDB | | Asarone, (Z)-isomer | HMDB | | cis-Isoasarone | HMDB | | trans-Asarone | HMDB | | gamma-Asarone | KEGG |
|
|---|
| Chemical Formula | C12H16O3 |
|---|
| Average Molecular Mass | 208.254 g/mol |
|---|
| Monoisotopic Mass | 208.110 g/mol |
|---|
| CAS Registry Number | 5353-15-1 |
|---|
| IUPAC Name | 1,2,4-trimethoxy-5-(prop-2-en-1-yl)benzene |
|---|
| Traditional Name | 1,2,4-trimethoxy-5-(prop-2-en-1-yl)benzene |
|---|
| SMILES | COC1=CC(OC)=C(OC)C=C1CC=C |
|---|
| InChI Identifier | InChI=1S/C12H16O3/c1-5-6-9-7-11(14-3)12(15-4)8-10(9)13-2/h5,7-8H,1,6H2,2-4H3 |
|---|
| InChI Key | AUNAUZZQBAIQFJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anisoles. These are organic compounds containing a methoxybenzene or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol ethers |
|---|
| Sub Class | Anisoles |
|---|
| Direct Parent | Anisoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenoxy compound
- Methoxybenzene
- Anisole
- Alkyl aryl ether
- Monocyclic benzene moiety
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-1900000000-4649f33292c90ff759e9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-6cf3105b3701707e31ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-3970000000-b406b55ae2fd9f8115ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-5900000000-c94fa7446dfec47971fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-92e05c63e4d5d2bb0ad2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4m-0930000000-02f7c263c6df8bfa6393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-3900000000-b9935bae41ab766f846b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-48e778bb33693fbfbece | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0390000000-78c563a7e431bf609152 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-8900000000-71c379564b68649ad6c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-65fdf834be31e8e78310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0970000000-62d17e234a9d063c72f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-9810000000-11edd9211ea1e5923104 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029872 |
|---|
| FooDB ID | FDB001101 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00042628 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 552473 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 636750 |
|---|
| Kegg Compound ID | C17821 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|