| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:05:37 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023488 |
|---|
| Identification |
|---|
| Common Name | Cochineal Red A |
|---|
| Class | Small Molecule |
|---|
| Description | Cochineal Red A is a synthetic food colouring. Because it is an azo dye, it may elicit intolerance in people allergic to salicylates (aspirin). Additionally, it is a histamine liberator, and may intensify symptoms of asthma. Ponceau 4R (also known as Food Red 7[citation needed], C.I. 16255[citation needed], Cochineal Red A[citation needed], New Coccine, Acid Red 18, SX purple[citation needed]) is a synthetic colourant that may be added to foods to induce a colour change. It is denoted by E Number E124, and has the capacity for inducing an allergic reaction. Its chemical name is trisodium salt of 1-(4-sulpho-1-napthylazo)- 2-napthol- 6,8-disulphonic acid. Ponceau 4R is a red azo dye usually synthesized from coal tar which can be used in a variety of food products |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
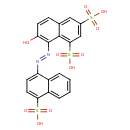
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7-Hydroxy-8-[(4-sulfO-1-naphthalenyl)azo]-1,3-naphthalenedisulfonic acid, 9ci | HMDB | | Acid scarlet 3R | HMDB | | Brilliant ponceau 3R | HMDB | | Brilliant scarlet 3R | HMDB | | C.I. 16255 | HMDB | | C.I. acid red 18 | HMDB | | C.I. FOOD red 7 | HMDB | | Coccine | HMDB | | e124 | HMDB | | FOOD Red no. 102 | HMDB | | Kayaku acid brilliant scarlet 3R | HMDB | | Naphthalene scarlet 4R | HMDB | | Neucoccin | HMDB | | New coccine | HMDB | | Ponceau 4R | HMDB | | Strawberry red | HMDB | | 7-Hydroxy-8-[(e)-2-(4-sulfonaphthalen-1-yl)diazen-1-yl]naphthalene-1,3-disulfonate | Generator | | 7-Hydroxy-8-[(e)-2-(4-sulphonaphthalen-1-yl)diazen-1-yl]naphthalene-1,3-disulphonate | Generator | | 7-Hydroxy-8-[(e)-2-(4-sulphonaphthalen-1-yl)diazen-1-yl]naphthalene-1,3-disulphonic acid | Generator |
|
|---|
| Chemical Formula | C20H14N2O10S3 |
|---|
| Average Molecular Mass | 538.528 g/mol |
|---|
| Monoisotopic Mass | 537.981 g/mol |
|---|
| CAS Registry Number | 7244-14-6 |
|---|
| IUPAC Name | 7-hydroxy-8-[(E)-2-(4-sulfonaphthalen-1-yl)diazen-1-yl]naphthalene-1,3-disulfonic acid |
|---|
| Traditional Name | 7-hydroxy-8-[(E)-2-(4-sulfonaphthalen-1-yl)diazen-1-yl]naphthalene-1,3-disulfonic acid |
|---|
| SMILES | OC1=C(\N=N\C2=CC=C(C3=CC=CC=C23)S(O)(=O)=O)C2=C(C=C(C=C2C=C1)S(O)(=O)=O)S(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C20H14N2O10S3/c23-16-7-5-11-9-12(33(24,25)26)10-18(35(30,31)32)19(11)20(16)22-21-15-6-8-17(34(27,28)29)14-4-2-1-3-13(14)15/h1-10,23H,(H,24,25,26)(H,27,28,29)(H,30,31,32)/b22-21+ |
|---|
| InChI Key | JZGWEIPJUAIDHM-QURGRASLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,1'-azonaphthalenes. These are organonitrogen aromatic compounds that contain a central azo group, where each nitrogen atom is conjugated to the 1-position of a naphthalene ring system. Naphthalene is a compound made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | 1,1'-azonaphthalenes |
|---|
| Direct Parent | 1,1'-azonaphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,1'-azonaphthalene
- 2-naphthalene sulfonate
- 1-naphthalene sulfonate
- Naphthalene sulfonic acid or derivatives
- 2-naphthalene sulfonic acid or derivatives
- 1-naphthalene sulfonic acid or derivatives
- Naphthalene sulfonate
- 2-naphthol
- Arylsulfonic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- 1-hydroxy-2-unsubstituted benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Azo compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organic oxide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0abj-0183930000-10173846688acf0aa460 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-2054290000-1d1406621f596b21cd3c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Cochineal Red A,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-0011290000-7442d4fcdafce7ee8dfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0022930000-1beb59e9fcdd60edb8d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0239200000-f6ec2416d228d5b9dd45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0022290000-162e0ddba488154d73ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-2045960000-fd35af997105419ac239 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0083-3392000000-0796c42aa02562b0f0a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0022090000-bc747b3cbb3d562ca22d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0093240000-d4c05d1a3be7d808d4c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-1931110000-1b931b40476b5e84ab94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000090000-20f074deed63688606cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-8000090000-2e6b66829f3c97c2f523 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9712000000-7aabbdbe4b66025e61a1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029855 |
|---|
| FooDB ID | FDB001076 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ponceau 4R |
|---|
| Chemspider ID | 10807381 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|