| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:05:08 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023478 |
|---|
| Identification |
|---|
| Common Name | Vulgaxanthin II |
|---|
| Class | Small Molecule |
|---|
| Description | Vulgaxanthin II is found in common beet. Vulgaxanthin II is a yellow pigment from beetroot (Beta vulgaris |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
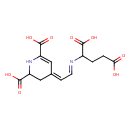
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-[[(1,3-Dicarboxypropyl)imino]ethylidene]-1,2,3,4-tetrahydro-2,6-pyridinedicarboxylic acid, 9ci | HMDB | | Vulgaxanthin-II | HMDB | | (4Z)-4-[(2E)-2-[(1,3-Dicarboxypropyl)imino]ethylidene]-1,2,3,4-tetrahydropyridine-2,6-dicarboxylate | Generator |
|
|---|
| Chemical Formula | C14H16N2O8 |
|---|
| Average Molecular Mass | 340.285 g/mol |
|---|
| Monoisotopic Mass | 340.091 g/mol |
|---|
| CAS Registry Number | 1047-87-6 |
|---|
| IUPAC Name | (4Z)-4-[(2E)-2-[(1,3-dicarboxypropyl)imino]ethylidene]-1,2,3,4-tetrahydropyridine-2,6-dicarboxylic acid |
|---|
| Traditional Name | (4Z)-4-[(2E)-2-[(1,3-dicarboxypropyl)imino]ethylidene]-2,3-dihydro-1H-pyridine-2,6-dicarboxylic acid |
|---|
| SMILES | OC(=O)CCC(\N=C\C=C1\CC(NC(=C1)C(O)=O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H16N2O8/c17-11(18)2-1-8(12(19)20)15-4-3-7-5-9(13(21)22)16-10(6-7)14(23)24/h3-5,8,10,16H,1-2,6H2,(H,17,18)(H,19,20)(H,21,22)(H,23,24)/b7-3+,15-4+ |
|---|
| InChI Key | POYIZOSTYKKRNT-XWKOYBCNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- Tetracarboxylic acid or derivatives
- Alpha-amino acid
- Tetrahydropyridine
- Hydropyridine
- Shiff base
- Amino acid
- Aldimine
- Carboxylic acid
- Secondary aliphatic amine
- Enamine
- Secondary amine
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Azacycle
- Organonitrogen compound
- Imine
- Organooxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-1391000000-ec9714f563a80800fb96 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-03di-2106095000-8bc67241d5158b0270b4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0097-0289000000-e317ebaad0c6349c6a57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6w-0981000000-2e12d921233777ebe34e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01pa-3980000000-e7b653c9a91e815df6c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000j-0379000000-53b8410f1a5bde8ef3fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0392000000-e60a7bcdf5e5400b8509 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udj-5930000000-417c4ffede37038b4da6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0097-0059000000-4e57190414a3fbff1bff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f92-0591000000-fb0d9eb73ab9d5ee9a06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-0920000000-5193d0962ea9e7b43b6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002k-0095000000-a4f81b509080249de152 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0092-1291000000-ad739eda011775381f81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fg5-3690000000-b92c381b7646cd09ece4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029841 |
|---|
| FooDB ID | FDB001056 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001608 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013089 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C08569 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|