| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:05:04 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023476 |
|---|
| Identification |
|---|
| Common Name | Ascorbigen |
|---|
| Class | Small Molecule |
|---|
| Description | Present in plants, especies cabbage and other crucifers. Ascorbigen is found in many foods, some of which are mung bean, guava, brassicas, and bitter gourd. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
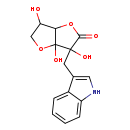
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-C-(1H-indol-3-Ylmethyl)-3-hexulofuranosonic acid g-lactone, 9ci | HMDB |
|
|---|
| Chemical Formula | C15H15NO6 |
|---|
| Average Molecular Mass | 305.283 g/mol |
|---|
| Monoisotopic Mass | 305.090 g/mol |
|---|
| CAS Registry Number | 8075-98-7 |
|---|
| IUPAC Name | 3,3a,6-trihydroxy-3-(1H-indol-3-ylmethyl)-hexahydrofuro[3,2-b]furan-2-one |
|---|
| Traditional Name | 3,3a,6-trihydroxy-3-(1H-indol-3-ylmethyl)-dihydro-5H-furo[3,2-b]furan-2-one |
|---|
| SMILES | OC1COC2(O)C1OC(=O)C2(O)CC1=CNC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C15H15NO6/c17-11-7-21-15(20)12(11)22-13(18)14(15,19)5-8-6-16-10-4-2-1-3-9(8)10/h1-4,6,11-12,16-17,19-20H,5,7H2 |
|---|
| InChI Key | OMSJCIOTCFHSIT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isosorbides. These are organic polycyclic compounds containing an isosorbide(1,4-Dianhydrosorbitol) moiety, which consists of two -oxolan-3-ol rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furofurans |
|---|
| Sub Class | Isosorbides |
|---|
| Direct Parent | Isosorbides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isosorbide
- 3-alkylindole
- Indole
- Indole or derivatives
- Gamma butyrolactone
- Monosaccharide
- Substituted pyrrole
- Benzenoid
- Heteroaromatic compound
- Tetrahydrofuran
- Tertiary alcohol
- Pyrrole
- Carboxylic acid ester
- Hemiacetal
- Secondary alcohol
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Polyol
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9640000000-ebafd13f557941194a24 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0040-8290400000-85ab052d313cd85c1fcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0859000000-1820214eaab9fa8999d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-ef82110695953a06c772 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2900000000-e7ad584b1157d19a0f1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0umi-1904000000-fe95df475a0a16900240 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aec-2900000000-4fc178aaf38e9bbfca30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9600000000-aaa0f976bcdd3b278871 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029839 |
|---|
| FooDB ID | FDB001054 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 317840 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 358022 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|