| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:05:03 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023475 |
|---|
| Identification |
|---|
| Common Name | Harmalol |
|---|
| Class | Small Molecule |
|---|
| Description | A harmala alkaloid in which the harman skeleton is hydroxy-substituted at C-7 and has been reduced across the 3,4 bond. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
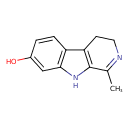
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methyl-4,9-dihydro-3H-beta-carbolin-7-ol | ChEBI | | Harmidol | ChEBI | | Harmolol | ChEBI | | 1-Methyl-4,9-dihydro-3H-b-carbolin-7-ol | Generator | | 1-Methyl-4,9-dihydro-3H-β-carbolin-7-ol | Generator | | 1-Methyl-4,9-dihydro-3H-beta-carbolin-7-ol hydrochloride | HMDB | | 3,4-dihydro-1-Methyl-2H-pyrido[3,4-b]indol-7-ol | HMDB | | 3,4-dihydro-7-Hydroxy-1-methyl-b-carboline | HMDB | | 4,9-dihydro-1-Methyl-3H-pyrido(3,4-b)indol-7-ol | HMDB | | 4,9-dihydro-1-Methyl-3H-pyrido[3,4-b]indol-7-ol | HMDB | | 4,9-dihydro-1-Methyl-3H-pyrido[3,4-b]indol-7-ol, 9ci | HMDB | | Harmalol hydrochloride | MeSH | | Harmalol hydrochloride, dihydrate | MeSH | | Harmalol trihydrate | MeSH | | Harmalol lactate, dihydrate | MeSH | | Harmalol dihydrochloride | MeSH |
|
|---|
| Chemical Formula | C12H12N2O |
|---|
| Average Molecular Mass | 200.237 g/mol |
|---|
| Monoisotopic Mass | 200.095 g/mol |
|---|
| CAS Registry Number | 525-57-5 |
|---|
| IUPAC Name | 1-methyl-3H,4H,9H-pyrido[3,4-b]indol-7-ol |
|---|
| Traditional Name | harmalol |
|---|
| SMILES | CC1=NCCC2=C1NC1=C2C=CC(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C12H12N2O/c1-7-12-10(4-5-13-7)9-3-2-8(15)6-11(9)14-12/h2-3,6,14-15H,4-5H2,1H3 |
|---|
| InChI Key | RHVPEFQDYMMNSY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harmaline
- Harmalol
- Harman
- Beta-carboline
- Pyridoindole
- Hydroxyindole
- 3-alkylindole
- Indole
- Indole or derivatives
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Ketimine
- Azacycle
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Imine
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-0900000000-e4464398b4dec3fc7352 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-7190000000-1284b3e28670778298fe | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0290000000-a117734dd274e28b35ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1970000000-b19698ef3a7ac01eec3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-0900000000-f10b93cb6c00a71896fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-aaba7e72d490321c1aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-78586bb5e1894bd774f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-2900000000-fd7e4b643b65563440d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-f12acafe48c5444229fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-f12acafe48c5444229fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06ea-0900000000-6f32f96c94e3e30167a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-02a2f360bd41f66f24a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0190000000-c702aa1aff1d9ef9f4a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-2900000000-8fe62270768d65d29f57 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029838 |
|---|
| FooDB ID | FDB001053 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00052310 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-9938 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Harmalol |
|---|
| Chemspider ID | 11262879 |
|---|
| ChEBI ID | 27943 |
|---|
| PubChem Compound ID | 3565 |
|---|
| Kegg Compound ID | C06537 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|