| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:04:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023472 |
|---|
| Identification |
|---|
| Common Name | Tetrahydroharmol |
|---|
| Class | Small Molecule |
|---|
| Description | Tetrahydroharmol is found in fruits. Tetrahydroharmol is an alkaloid from Elaeagnus angustifolia (Russian olive) Harmaline is a reversible inhibitor of MAO-A (RIMA). Harmine is a reversible inhibitor of MAO-A (RIMA). It is important to note that unlike synthetic pharmaceutical MAOIs such as phenelzine, harmine is reversible and selective meaning it does not have nearly as high a risk for the "cheese syndrome" caused by consuming tyramine-containing foods, which is a risk associated with monoamine oxidase A inhibitors, but not monoamine oxidase B inhibitors. Several alkaloids that function as monoamine oxidase inhibitors (MAOIs) are found in the seeds of Peganum harmala (also known as Harmal or Syrian Rue), including harmine, harmaline, and harmalol, which are members of a group of substances with a similar chemical structure collectively known as harmala alkaloids. These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. The harmala alkaloid harmine which was once known as Telepathine and Banisterine is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi, but not P. harmala. Dr. Alexander Shulgin has suggesed that harmaline may be a breakdown product of harmine. Harmine and harmaline are reversible MAOIs of the MAO-A isoform of the enzyme, and can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine. The harmala alkaloids occur in Peganum harmala in concentrations of roughly 3%, though tests have documented anywhere from 2-7%, as natural sources tend to vary widely in chemical makeup. Harmala alkaloids are also found in the Banisteriopsis caapi vine, the key plant ingredient in the sacramental beverage Ayahuasca, in concentrations that range between 0.31-8.43% for harmine, 0.03-0.83% for harmaline and 0.05-2.94% for tetrahydroharmine. Other psychoactive plants are often added to Ayahuasca to achieve visionary states of consciousness; for example leaves from Psychotria viridis, which is a source of dimethyltryptamine (DMT). The harmala alkaloids serve to potentiate these brewed compounds by preventing their breakdown in the digestive tract. The harmala alkaloids are not especially psychoactive on their own, even at high dosages, when vomiting and diarrhea become the main effect |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
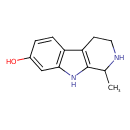
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,4-Tetrahydro-7-hydroxy-1-methyl-b-carboline | HMDB | | 2,3,4,9-Tetrahydro-1-methyl-1H-pyrido[3,4-b]indol-7-ol, 8ci | HMDB | | Tetrahydroharmalol hydrochloride | HMDB | | Tetrahydroharmol hydrochloride | HMDB |
|
|---|
| Chemical Formula | C12H14N2O |
|---|
| Average Molecular Mass | 202.252 g/mol |
|---|
| Monoisotopic Mass | 202.111 g/mol |
|---|
| CAS Registry Number | 17952-75-9 |
|---|
| IUPAC Name | 1-methyl-1H,2H,3H,4H,9H-pyrido[3,4-b]indol-7-ol |
|---|
| Traditional Name | 1-methyl-1H,2H,3H,4H,9H-pyrido[3,4-b]indol-7-ol |
|---|
| SMILES | CC1NCCC2=C1NC1=C2C=CC(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C12H14N2O/c1-7-12-10(4-5-13-7)9-3-2-8(15)6-11(9)14-12/h2-3,6-7,13-15H,4-5H2,1H3 |
|---|
| InChI Key | AZTMWIPCEFFOJD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harmaline
- Harmalol
- Harman
- Beta-carboline
- Pyridoindole
- Hydroxyindole
- 3-alkylindole
- Indole
- Indole or derivatives
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Organoheterocyclic compound
- Azacycle
- Secondary aliphatic amine
- Secondary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-059i-0910000000-70bfd23f847e808c0f3e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0ab9-3690000000-b50240f2dfd89efd370a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0190000000-dbf0ba828934a3d16b85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w29-1950000000-cd07495fb3fab4274fb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-0900000000-a9ba3cd5545cb553e8b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-54e3ee3f4a7697515db6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-ffda0c873192bf93a9c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ac9-2900000000-8947f0a5c18fbf6a5813 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-53c3fa4c5460be246d0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-53c3fa4c5460be246d0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053s-0900000000-a644d6e5d295344f8a9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-ee2fb06e46c3edc2e07a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0190000000-85a10545fbc1fd164f55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g0-2900000000-4bc929863d4e520a914d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029835 |
|---|
| FooDB ID | FDB001050 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057972 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetrahydroharmol |
|---|
| Chemspider ID | 327573 |
|---|
| ChEBI ID | 114202 |
|---|
| PubChem Compound ID | 368982 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|