| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:04:11 UTC |
|---|
| Update Date | 2016-11-09 01:17:43 UTC |
|---|
| Accession Number | CHEM023450 |
|---|
| Identification |
|---|
| Common Name | Phytosulfokine a |
|---|
| Class | Small Molecule |
|---|
| Description | Phytosulfokine a is found in green vegetables. Phytosulfokine a is a constituent of asparagus spears (Asparagus officinalis) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
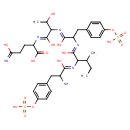
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Phytosulphokine a | Generator | | 2-({2-[(2-{[2-({2-amino-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene}amino)-1-hydroxy-3-methylpentylidene]amino}-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene)amino]-1,3-dihydroxybutylidene}amino)-4-(C-hydroxycarbonimidoyl)butanoate | HMDB | | 2-({2-[(2-{[2-({2-amino-1-hydroxy-3-[4-(sulphooxy)phenyl]propylidene}amino)-1-hydroxy-3-methylpentylidene]amino}-1-hydroxy-3-[4-(sulphooxy)phenyl]propylidene)amino]-1,3-dihydroxybutylidene}amino)-4-(C-hydroxycarbonimidoyl)butanoate | HMDB | | 2-({2-[(2-{[2-({2-amino-1-hydroxy-3-[4-(sulphooxy)phenyl]propylidene}amino)-1-hydroxy-3-methylpentylidene]amino}-1-hydroxy-3-[4-(sulphooxy)phenyl]propylidene)amino]-1,3-dihydroxybutylidene}amino)-4-(C-hydroxycarbonimidoyl)butanoic acid | HMDB |
|
|---|
| Chemical Formula | C33H46N6O16S2 |
|---|
| Average Molecular Mass | 846.879 g/mol |
|---|
| Monoisotopic Mass | 846.241 g/mol |
|---|
| CAS Registry Number | 179667-62-0 |
|---|
| IUPAC Name | 2-[(Z)-{2-[(Z)-{2-[(Z)-{2-[(Z)-{2-amino-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene}amino]-1-hydroxy-3-methylpentylidene}amino]-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene}amino]-1,3-dihydroxybutylidene}amino]-4-(C-hydroxycarbonimidoyl)butanoic acid |
|---|
| Traditional Name | 2-[(Z)-{2-[(Z)-{2-[(Z)-{2-[(Z)-{2-amino-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene}amino]-1-hydroxy-3-methylpentylidene}amino]-1-hydroxy-3-[4-(sulfooxy)phenyl]propylidene}amino]-1,3-dihydroxybutylidene}amino]-4-(C-hydroxycarbonimidoyl)butanoic acid |
|---|
| SMILES | CCC(C)C(\N=C(/O)C(N)CC1=CC=C(OS(O)(=O)=O)C=C1)C(\O)=N\C(CC1=CC=C(OS(O)(=O)=O)C=C1)C(\O)=N\C(C(C)O)C(\O)=N\C(CCC(O)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C33H46N6O16S2/c1-4-17(2)27(38-29(42)23(34)15-19-5-9-21(10-6-19)54-56(48,49)50)31(44)37-25(16-20-7-11-22(12-8-20)55-57(51,52)53)30(43)39-28(18(3)40)32(45)36-24(33(46)47)13-14-26(35)41/h5-12,17-18,23-25,27-28,40H,4,13-16,34H2,1-3H3,(H2,35,41)(H,36,45)(H,37,44)(H,38,42)(H,39,43)(H,46,47)(H,48,49,50)(H,51,52,53) |
|---|
| InChI Key | BSUVNQLYZGXYQY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as peptides. Peptides are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Peptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha peptide
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Phenylsulfate
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Arylsulfate
- Phenoxy compound
- Aralkylamine
- Monocyclic benzene moiety
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Benzenoid
- Organic sulfuric acid or derivatives
- Amino acid or derivatives
- Secondary alcohol
- Amino acid
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic oxygen compound
- Primary aliphatic amine
- Amine
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ta-0341110190-dc6e510675d05017df9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2982210320-61d3102a426cf383c0e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-4693100000-97457a6f2c2dcfbd5315 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-0011003390-303200ea3e3032f7d037 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-1011011930-0887af9703a406a5f979 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9517322100-b218a880baa948d33bd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0145001290-8c3e70e10f493c01311f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00pi-7595122150-6b037b9686d0ebb2f6cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ks-2942000310-2bf46e012ec7afbe4bfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-0320113090-4792434871611e3a682a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-6922107000-8ef98974ce5fcbac0c95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9660010000-2ccadf919233820cd9f8 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029809 |
|---|
| FooDB ID | FDB001020 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057158 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013085 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 72795529 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|