| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:03:39 UTC |
|---|
| Update Date | 2016-11-09 01:17:42 UTC |
|---|
| Accession Number | CHEM023436 |
|---|
| Identification |
|---|
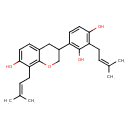
| Common Name | (R)-2',4',7-Trihydroxy-3',8-diprenylisoflavan |
|---|
| Class | Small Molecule |
|---|
| Description | (R)-2',4',7-Trihydroxy-3',8-diprenylisoflavan is found in herbs and spices. (R)-2',4',7-Trihydroxy-3',8-diprenylisoflavan is a constituent of Glycyrrhiza glabra (licorice) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3R)-7,2',4'-Trihydroxy-8,3'-diprenyloxyisoflavan | HMDB | | Kanzonol X | HMDB | | Tenuifolin b | HMDB |
|
|---|
| Chemical Formula | C25H30O4 |
|---|
| Average Molecular Mass | 394.503 g/mol |
|---|
| Monoisotopic Mass | 394.214 g/mol |
|---|
| CAS Registry Number | 182745-37-5 |
|---|
| IUPAC Name | 4-[7-hydroxy-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-1-benzopyran-3-yl]-2-(3-methylbut-2-en-1-yl)benzene-1,3-diol |
|---|
| Traditional Name | 4-[7-hydroxy-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-1-benzopyran-3-yl]-2-(3-methylbut-2-en-1-yl)benzene-1,3-diol |
|---|
| SMILES | CC(C)=CCC1=C(O)C=CC(C2COC3=C(CC=C(C)C)C(O)=CC=C3C2)=C1O |
|---|
| InChI Identifier | InChI=1S/C25H30O4/c1-15(2)5-8-20-22(26)12-10-19(24(20)28)18-13-17-7-11-23(27)21(9-6-16(3)4)25(17)29-14-18/h5-7,10-12,18,26-28H,8-9,13-14H2,1-4H3 |
|---|
| InChI Key | KZTSESJJLOEXBX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoflavanols. These are polycyclic compounds containing a hydroxylated isoflavan skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Isoflavans |
|---|
| Direct Parent | Isoflavanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoflavanol
- Hydroxyisoflavonoid
- Chromane
- Benzopyran
- 1-benzopyran
- Resorcinol
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Organoheterocyclic compound
- Ether
- Oxacycle
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fbl-2019000000-da6c277eaae9830dd50f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0002-1000090000-8f585202108bc12fab05 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000g-0918000000-bfe672cd246972328a59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014u-2965000000-2773837bb1a195fdfbd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-4933000000-dc25fa47473c0bb1c676 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0219000000-d618f53fea88f2605689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ou-0977000000-9e8592e35572a4d64b63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002r-1931000000-2402517cc60319e5ec3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-fba1306000e9686a2aae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0009000000-d1147ab28b4ee9a43e5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0937000000-78f89fa5188ce325a682 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-0298000000-54914f995e153fba2cf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1479000000-97d7fa9836f21470cc50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-2983000000-6f355886d653a77c281f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029793 |
|---|
| FooDB ID | FDB001001 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019467 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 29332019 |
|---|
| ChEBI ID | 172632 |
|---|
| PubChem Compound ID | 15380911 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|