| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:01:37 UTC |
|---|
| Update Date | 2016-11-09 01:17:42 UTC |
|---|
| Accession Number | CHEM023394 |
|---|
| Identification |
|---|
| Common Name | 3-(1H-Indol-3-yl)-2-propenoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | An alpha,beta-unsaturated monocarboxylic acid that is acrylic acid in which one of the hydrogens at position 3 is replaced by an indol-2-yl group. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
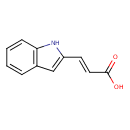
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Indoleacrylic acid | ChEBI | | 2-Indolylacrylic acid | ChEBI | | 3-(2-Indolyl)acrylic acid | ChEBI | | Indole-2-acrylic acid | ChEBI | | trans-2-Indoleacrylic acid | ChEBI | | 2-Indoleacrylate | Generator | | 2-Indolylacrylate | Generator | | 3-(2-Indolyl)acrylate | Generator | | Indole-2-acrylate | Generator | | trans-2-Indoleacrylate | Generator | | Indoleacrylate | Generator | | (e)-3-(indol-2-yl)Acrylate | HMDB | | 3-Indoleacrylate | HMDB | | 3-Indoleacrylic acid | HMDB | | Indoleacrylic acid | ChEBI |
|
|---|
| Chemical Formula | C11H9NO2 |
|---|
| Average Molecular Mass | 187.195 g/mol |
|---|
| Monoisotopic Mass | 187.063 g/mol |
|---|
| CAS Registry Number | 1204-06-4 |
|---|
| IUPAC Name | (2E)-3-(1H-indol-2-yl)prop-2-enoic acid |
|---|
| Traditional Name | (2E)-3-(1H-indol-2-yl)prop-2-enoic acid |
|---|
| SMILES | OC(=O)\C=C/C1=CNC2=C1C=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C11H9NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-7,12H,(H,13,14)/b6-5- |
|---|
| InChI Key | PLVPPLCLBIEYEA-WAYWQWQTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indoles. Indoles are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indoles |
|---|
| Direct Parent | Indoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indole
- Substituted pyrrole
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-1900000000-5bea0c7cc28fbf16cc9b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-8930000000-dd31397a073d8d298f0b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0900000000-1e4da4a40a13dc3040ce | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-02tc-0900000000-185cb8e2bf411f93c23f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014l-9700000000-db32fca92b926fe968e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-f69f85afb3ba3821b065 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0900000000-49227cba19b681b92792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-4900000000-c34f2b3621590f6fe3df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-04cbdafbbbb963000ee9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ku-0900000000-89a52e5e9945e195469d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-0900000000-dceb7d5d8c109f075486 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000734 |
|---|
| FooDB ID | FDB000938 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000111 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5702 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10607876 |
|---|
| ChEBI ID | 90333 |
|---|
| PubChem Compound ID | 15030923 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Moffatt, J. S. Preparation of b-3-indolylacrylic acid. Journal of the Chemical Society (1957), 1442-3.; Bauguess, Lyle C.; Berg, Clarence P. The availability of indole derivatives for supplementing diets deficient in tryptophan. Proceedings of the Iowa Academy of Science (1933), 40 110-11. | | 2. Moffatt, J. S. Preparation of b-3-indolylacrylic acid. Journal of the Chemical Society (1957), 1442-3.; Bauguess, Lyle C.; Berg, Clarence P. The availability of indole derivatives for supplementing diets deficient in tryptophan. Proceedings of the Iowa Academy of Science (1933), 40 110-11. | | 3. Marklova E: Where does indolylacrylic acid come from? Amino Acids. 1999;17(4):401-13. | | 4. Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O'Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ: Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe. 2017 Jul 12;22(1):25-37.e6. doi: 10.1016/j.chom.2017.06.007. | | 5. Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL: A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017 Nov 30;551(7682):648-652. doi: 10.1038/nature24661. Epub 2017 Nov 22. |
|
|---|