| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:57:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:40 UTC |
|---|
| Accession Number | CHEM023285 |
|---|
| Identification |
|---|
| Common Name | (4-Hydroxybenzoyl)choline |
|---|
| Class | Small Molecule |
|---|
| Description | (4-Hydroxybenzoyl)choline is found in herbs and spices. (4-Hydroxybenzoyl)choline is an alkaloid from white mustard (Sinapis alba |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
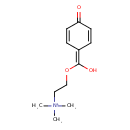
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-((4-Hydroxybenzoyl)oxy)-N,N,N-trimethyl-ethanaminium | HMDB | | 4-Hydroxybenzoylcholine | HMDB | | Para-hydroxybenzoylcholine | HMDB | | Parabens-choline | HMDB | | p-Hydroxybenzoylcholine bisulfate | HMDB |
|
|---|
| Chemical Formula | C12H18NO3 |
|---|
| Average Molecular Mass | 224.276 g/mol |
|---|
| Monoisotopic Mass | 224.129 g/mol |
|---|
| CAS Registry Number | 5094-31-5 |
|---|
| IUPAC Name | {2-[hydroxy(4-oxocyclohexa-2,5-dien-1-ylidene)methoxy]ethyl}trimethylazanium |
|---|
| Traditional Name | {2-[hydroxy(4-oxocyclohexa-2,5-dien-1-ylidene)methoxy]ethyl}trimethylazanium |
|---|
| SMILES | C[N+](C)(C)CCOC(O)=C1C=CC(=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C12H17NO3/c1-13(2,3)8-9-16-12(15)10-4-6-11(14)7-5-10/h4-7H,8-9H2,1-3H3/p+1 |
|---|
| InChI Key | BAPAICNRGIBFJT-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as p-hydroxybenzoic acid alkyl esters. These are aromatic compounds containing a benzoic acid, which is esterified with an alkyl group and para-substituted with a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | p-Hydroxybenzoic acid alkyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-hydroxybenzoic acid alkyl ester
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Amine
- Organic nitrogen compound
- Organic salt
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic cation
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-9620000000-87ca96d74f5a4f8d723f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dr-9130000000-19331728140232a521b3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00xr-0890000000-73b6957acc0721cd372a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-014i-0900000000-e54b6698097ec9ced95b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0900000000-3c8dce9d9b88dcc4be6f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-7900000000-948cf79f530574c15b5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-2290000000-a1d6a71bbaf148ed706f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-4950000000-69f348f4c705e27a6f2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00wi-9200000000-23ee7c46b2b51ed6dd13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00ei-6940000000-3997a6640dc51d919038 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-6910000000-431cc407f0fc00226a29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0229-9500000000-a1e85e4645f00430a04e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029559 |
|---|
| FooDB ID | FDB000712 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 133307 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 151252 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|