| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:55:12 UTC |
|---|
| Update Date | 2016-11-09 01:17:39 UTC |
|---|
| Accession Number | CHEM023234 |
|---|
| Identification |
|---|
| Common Name | Laccaic acid D |
|---|
| Class | Small Molecule |
|---|
| Description | A trihydroxyanthraquinone that is that is 3,6,8-trihydroxy-9,10-anthraquinone substituted by methyl and carboxy groups at positions 1 and 2 respectively. A minor component of LAC dye together with laccaic acids A, B and C. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
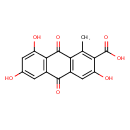
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 9,10-Dihydro-3,6,8-trihydroxy-1-methyl-9,10-dioxo-2-anthracenecarboxylic acid | ChEBI | | Flavokermesic acid | ChEBI | | Xanthokermesic acid | ChEBI | | 9,10-Dihydro-3,6,8-trihydroxy-1-methyl-9,10-dioxo-2-anthracenecarboxylate | Generator | | Flavokermesate | Generator | | Xanthokermesate | Generator | | Laccaate D | Generator | | 9,10-Dihydro-3,6,8-trihydroxy-1-methyl-9,10-dioxo-2-anthracenecarboxylic acid, 9ci | HMDB |
|
|---|
| Chemical Formula | C16H10O7 |
|---|
| Average Molecular Mass | 314.246 g/mol |
|---|
| Monoisotopic Mass | 314.043 g/mol |
|---|
| CAS Registry Number | 18499-84-8 |
|---|
| IUPAC Name | 3,6,8-trihydroxy-1-methyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid |
|---|
| Traditional Name | 3,6,8-trihydroxy-1-methyl-9,10-dioxoanthracene-2-carboxylic acid |
|---|
| SMILES | CC1=C(C(O)=O)C(O)=CC2=C1C(=O)C1=C(C=C(O)C=C1O)C2=O |
|---|
| InChI Identifier | InChI=1S/C16H10O7/c1-5-11-8(4-10(19)12(5)16(22)23)14(20)7-2-6(17)3-9(18)13(7)15(11)21/h2-4,17-19H,1H3,(H,22,23) |
|---|
| InChI Key | DDTNCHWMNZLWKO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthracenecarboxylic acids. These are organic compounds containing a carboxylic acid group attached to an anthracene ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthracenecarboxylic acids and derivatives |
|---|
| Direct Parent | Anthracenecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracene carboxylic acid
- 9,10-anthraquinone
- Anthraquinone
- Hydroxyanthraquinone
- 2-naphthalenecarboxylic acid
- 2-naphthalenecarboxylic acid or derivatives
- Salicylic acid or derivatives
- Hydroxybenzoic acid
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Vinylogous acid
- Ketone
- Monocarboxylic acid or derivatives
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02ds-0491000000-1358b194a0c9351ccb98 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-05tr-2202190000-65c1b7258bee80c66ba9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0049000000-ffd6441f57a0b87b0922 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0094000000-b793451cac47f1a57676 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2590000000-cdace83113f5c43ec5dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0069000000-d71f14e1a742275501af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0091000000-c72b244aa19ca6d9de13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-0290000000-fcfd0ee63ab61408da2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0098000000-65fbab827b8177985dbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0091000000-e73d5be536d73183f918 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-0090000000-f99d06f1f8a3e9b7d4b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0095000000-9e34f0b963f3809e8c16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-e32a154b20754d0f2984 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0290000000-75ccc2ef75c2baf21011 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029508 |
|---|
| FooDB ID | FDB000643 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00051186 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8058979 |
|---|
| ChEBI ID | 90194 |
|---|
| PubChem Compound ID | 9883304 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|