| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:54:50 UTC |
|---|
| Update Date | 2016-11-09 01:17:39 UTC |
|---|
| Accession Number | CHEM023225 |
|---|
| Identification |
|---|
| Common Name | 3'-Methoxyfukiic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 3'-Methoxyfukiic acid is found in green vegetables. 3'-Methoxyfukiic acid is isolated from Petasites japonicus (sweet coltsfoot |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
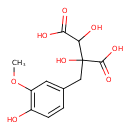
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Methoxyfukiate | Generator | | 2,3-Dihydroxy-2-[(4-hydroxy-3-methoxyphenyl)methyl]butanedioate | HMDB |
|
|---|
| Chemical Formula | C12H14O8 |
|---|
| Average Molecular Mass | 286.235 g/mol |
|---|
| Monoisotopic Mass | 286.069 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2,3-dihydroxy-2-[(4-hydroxy-3-methoxyphenyl)methyl]butanedioic acid |
|---|
| Traditional Name | 2,3-dihydroxy-2-[(4-hydroxy-3-methoxyphenyl)methyl]butanedioic acid |
|---|
| SMILES | COC1=C(O)C=CC(CC(O)(C(O)C(O)=O)C(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C12H14O8/c1-20-8-4-6(2-3-7(8)13)5-12(19,11(17)18)9(14)10(15)16/h2-4,9,13-14,19H,5H2,1H3,(H,15,16)(H,17,18) |
|---|
| InChI Key | DDSGTDZCSPMYIM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenylpropanoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylpropanoic-acid
- Methoxyphenol
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Beta-hydroxy acid
- Phenol
- Alpha-hydroxy acid
- Benzenoid
- Monosaccharide
- Hydroxy acid
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Tertiary alcohol
- Secondary alcohol
- 1,2-diol
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014l-6950000000-01646ee193b586ff250e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-003r-6010479000-cd3c8ec01280f05480cc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0390000000-1ed2353b39d7b9e1d9a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-3940000000-3233e48fa711dacfb886 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-2900000000-917655bcf695190a2c2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-08fu-2690000000-eb74af57a4c23d70aae3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07r1-3930000000-c6ac2ef6baaad477b036 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05vk-7900000000-a139ba46645bac07eed3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01xw-2790000000-3118fa65768341a7ac61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmi-2910000000-1fb33fc114503d726ac8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-008d-4940000000-9703d5288f8761aaedf1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-55ce5ff8a33f2c897993 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-1900000000-e87075077d5e865a7cec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-2900000000-8e43f7f805b4c229085d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029498 |
|---|
| FooDB ID | FDB000631 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013063 |
|---|
| ChEBI ID | 172518 |
|---|
| PubChem Compound ID | 131750880 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|