| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:52:23 UTC |
|---|
| Update Date | 2016-11-09 01:17:39 UTC |
|---|
| Accession Number | CHEM023158 |
|---|
| Identification |
|---|
| Common Name | Penmacric acid |
|---|
| Class | Small Molecule |
|---|
| Description | Penmacric acid is a constituent of the seeds of the famine feed Pentaclethra macrophylla |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
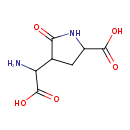
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Penmacrate | Generator | | 3-(1-Aminocarboxymethyl)-2-pyrrolidone-5-carboxylic acid | HMDB | | a-Amino-5-carboxy-2-oxo-3-pyrrolidineacetic acid, 9ci | HMDB | | 4-[Amino(carboxy)methyl]-5-hydroxy-3,4-dihydro-2H-pyrrole-2-carboxylate | HMDB | | Penmacric acid | MeSH |
|
|---|
| Chemical Formula | C7H10N2O5 |
|---|
| Average Molecular Mass | 202.165 g/mol |
|---|
| Monoisotopic Mass | 202.059 g/mol |
|---|
| CAS Registry Number | 55297-13-7 |
|---|
| IUPAC Name | 4-[amino(carboxy)methyl]-5-oxopyrrolidine-2-carboxylic acid |
|---|
| Traditional Name | 4-[amino(carboxy)methyl]-5-oxopyrrolidine-2-carboxylic acid |
|---|
| SMILES | NC(C1CC(NC1=O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H10N2O5/c8-4(7(13)14)2-1-3(6(11)12)9-5(2)10/h2-4H,1,8H2,(H,9,10)(H,11,12)(H,13,14) |
|---|
| InChI Key | QEFCFJFZZLNSPP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid
- Oxoproline
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Dicarboxylic acid or derivatives
- Pyrrolidone
- 2-pyrrolidone
- Pyrrolidine
- Carboxamide group
- Lactam
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Amine
- Organic oxide
- Primary amine
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0btc-7900000000-f0c0a520d46e0d9aece7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9444000000-3c7179cd697bf4eb6797 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2970000000-b9a25352f198cb20d40b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00e9-7910000000-b74949b56d8634b250a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-008c-9300000000-f68d59acd2ce97d155a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1790000000-96dad59716dce53571df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fai-7920000000-64b4b807d7d06bd53cb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-5fc4820445e40630b47c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0910000000-3600072b9506a8a1bde8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-1900000000-a2d5df2e0c14e36c89fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-9800000000-0beb1edaa0123037564b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zg0-0940000000-fe1282e64d0f4785a9ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-4900000000-1a4e8cc602f25efc64e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9300000000-36a9ff50dde59065a002 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029436 |
|---|
| FooDB ID | FDB000541 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054402 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 471952 |
|---|
| ChEBI ID | 167964 |
|---|
| PubChem Compound ID | 5231917 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|