| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023143 |
|---|
| Identification |
|---|
| Common Name | N-Acetyldjenkolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | N-Acetyldjenkolic acid is isolated from Acacia farnesiana (sweet acacia |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
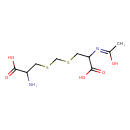
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Acetyldjenkolate | Generator | | 2-Amino-3-{[({2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl}sulfanyl)methyl]sulfanyl}propanoate | HMDB | | 2-Amino-3-{[({2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl}sulphanyl)methyl]sulphanyl}propanoate | HMDB | | 2-Amino-3-{[({2-carboxy-2-[(1-hydroxyethylidene)amino]ethyl}sulphanyl)methyl]sulphanyl}propanoic acid | HMDB |
|
|---|
| Chemical Formula | C9H16N2O5S2 |
|---|
| Average Molecular Mass | 296.364 g/mol |
|---|
| Monoisotopic Mass | 296.050 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-amino-3-{[({2-carboxy-2-[(Z)-(1-hydroxyethylidene)amino]ethyl}sulfanyl)methyl]sulfanyl}propanoic acid |
|---|
| Traditional Name | 2-amino-3-{[({2-carboxy-2-[(Z)-(1-hydroxyethylidene)amino]ethyl}sulfanyl)methyl]sulfanyl}propanoic acid |
|---|
| SMILES | C\C(O)=N\C(CSCSCC(N)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H16N2O5S2/c1-5(12)11-7(9(15)16)3-18-4-17-2-6(10)8(13)14/h6-7H,2-4,10H2,1H3,(H,11,12)(H,13,14)(H,15,16) |
|---|
| InChI Key | KXLLUVVNYPFZTI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Cysteine or derivatives
- Alpha-amino acid
- Dicarboxylic acid or derivatives
- Thioacetal
- Acetamide
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic nitrogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9130000000-69537fe5bcefceef2b85 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-9312200000-bfe99aa1aceb18b82a78 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uea-3590000000-223cb8688b726dd2a319 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-4910000000-672cd6a99747c5959cb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-9610000000-6adcc3dee73226d87c76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-06ed-3950000000-4bd9ff5c5939735a29cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-044i-4920000000-acf3235d3f10614af41f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004r-9200000000-c60c86d13538f01a4dfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0590000000-d178ac619d4da11fa656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-008i-4900000000-35c7651062355ea22f35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00vi-9500000000-8c1e8f1aaf811350f6c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9720000000-2d047509a37de75c9de1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-d0241e8ab1cec019b815 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-0cc29547ff5088cc471a | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029421 |
|---|
| FooDB ID | FDB000519 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35032868 |
|---|
| ChEBI ID | 174810 |
|---|
| PubChem Compound ID | 78061794 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|