| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:51:35 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023139 |
|---|
| Identification |
|---|
| Common Name | L-Targinine |
|---|
| Class | Small Molecule |
|---|
| Description | A L-arginine derivative with a N(omega)-methyl substituent. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
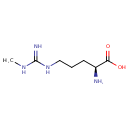
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acide (2S)-2-amino-5-(3-methylguanidino)pentanoique | ChEBI | | L-Monomethylarginine | ChEBI | | L-NMMA | ChEBI | | N-Monomethyl-L-arginine | ChEBI | | N(5)-(Methylamidino)-L-ornithine | ChEBI | | N(5)-(Metilamidino)-L-ornitina | ChEBI | | N(g)-Monomethyl-L-arginine | ChEBI | | Ngamma-monomethyl-L-arginine | ChEBI | | Omega-N-methylarginine | ChEBI | | Omega-N-monomethylarginine | ChEBI | | Targinina | ChEBI | | Targinine | ChEBI | | Targininum | ChEBI | | Tilarginina | ChEBI | | Tilarginine | ChEBI | | Tilargininum | ChEBI | | N(5)-(N-Methylcarbamimidoyl)-L-ornithine | HMDB | | N(5)-[Imino(methylamino)methyl]-L-ornithine | HMDB | | N(Omega)-methyl-L-arginine | HMDB | | N-Methyl-L-arginine | HMDB | | N-Omega-methyl-L-arginine | HMDB | | N-Omega-monomethyl-L-arginine | HMDB | | N5-(N-Methylcarbamimidoyl)-L-ornithine | HMDB | | NG-Monomethyl-L-argine | HMDB | | L NG Monomethyl arginine | HMDB | | NG Monomethyl L arginine | HMDB | | Omega N methylarginine | HMDB | | Arginine, L-NG-monomethyl | HMDB | | N(g)-Methylarginine | HMDB | | N(g)-Monomethyl-D-arginine | HMDB | | N(g)-Monomethylarginine | HMDB | | N(Omega)-monomethyl-L-arginine | HMDB | | D-NMMA | HMDB | | L Monomethylarginine | HMDB | | L-NG-Monomethyl arginine | HMDB | | NG-Monomethyl-L-arginine | HMDB |
|
|---|
| Chemical Formula | C7H16N4O2 |
|---|
| Average Molecular Mass | 188.228 g/mol |
|---|
| Monoisotopic Mass | 188.127 g/mol |
|---|
| CAS Registry Number | 17035-90-4 |
|---|
| IUPAC Name | (2S)-2-amino-5-(3-methylcarbamimidamido)pentanoic acid |
|---|
| Traditional Name | NG monomethyl L arginine |
|---|
| SMILES | CNC(=N)NCCC[C@H](N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 |
|---|
| InChI Key | NTNWOCRCBQPEKQ-YFKPBYRVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as arginine and derivatives. Arginine and derivatives are compounds containing arginine or a derivative thereof resulting from reaction of arginine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Arginine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Arginine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Fatty acid
- Guanidine
- Amino acid
- Carboximidamide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Imine
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00du-9300000000-8a5d10c2c8d6cd8eae8e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dr-9210000000-c205856837ac75413884 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-00di-9400000000-3cdd79f488639c85a738 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0076-1900000000-b304f22c29cac910afdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-9800000000-e1e925df5f5a69746f04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-23540f46e00006c9651c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2900000000-87d59411066c5f0db9e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9700000000-a2bcbbf60caf5fc66643 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9000000000-0cc6ad3e3fb7ee0fb63d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-3900000000-4a2c96ac18bbeebdfa44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-0e534ead44193d591ee2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-ff195acbbdd550fee9d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-a7a55bed1d490bf8ce9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1900000000-608a283cd867c13b9c94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-023ee0e0eb4f261fe594 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11815 |
|---|
| HMDB ID | HMDB0029416 |
|---|
| FooDB ID | FDB000510 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | NMM |
|---|
| Wikipedia Link | Methylarginine |
|---|
| Chemspider ID | 117259 |
|---|
| ChEBI ID | 28229 |
|---|
| PubChem Compound ID | 132862 |
|---|
| Kegg Compound ID | C03884 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. | | 2. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|