| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:51:20 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023132 |
|---|
| Identification |
|---|
| Common Name | Turicine |
|---|
| Class | Small Molecule |
|---|
| Description | An amino-acid betaine that is trans-4-hydroxy-D-proline zwitterion in which both of the hydrogens attached to the nitrogen have been replaced by methyl groups. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
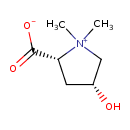
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Turicine | ChEBI | | Combretin a | HMDB | | Turicine | ChEBI |
|
|---|
| Chemical Formula | C7H13NO3 |
|---|
| Average Molecular Mass | 159.183 g/mol |
|---|
| Monoisotopic Mass | 159.090 g/mol |
|---|
| CAS Registry Number | 515-24-2 |
|---|
| IUPAC Name | (2R,4R)-4-hydroxy-1,1-dimethylpyrrolidin-1-ium-2-carboxylate |
|---|
| Traditional Name | (2R,4R)-4-hydroxy-1,1-dimethylpyrrolidin-1-ium-2-carboxylate |
|---|
| SMILES | C[N+]1(C)C[C@H](O)C[C@@H]1C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C7H13NO3/c1-8(2)4-5(9)3-6(8)7(10)11/h5-6,9H,3-4H2,1-2H3/t5-,6-/m1/s1 |
|---|
| InChI Key | MUNWAHDYFVYIKH-PHDIDXHHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid
- D-alpha-amino acid
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- N-alkylpyrrolidine
- Tetraalkylammonium salt
- Pyrrolidine
- Quaternary ammonium salt
- 1,2-aminoalcohol
- Carboxylic acid salt
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Organopnictogen compound
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic salt
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-9100000000-50c5b1c24168d0a32658 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0629-6900000000-39b7181df315007ea028 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0900000000-6a9f0e030e0567378c5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-4900000000-442e22b36ad486b30db5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ai-9000000000-c8419ca7ef208db583d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-067ab41a06b72183a015 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1900000000-ddc27ef204deb01df6bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pi3-9000000000-8f4fc7a9a1b02c966f3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-bae995f985c28e19f14c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-bae995f985c28e19f14c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-8900000000-bef7213e00adbdebf43f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1900000000-cdf52381a0c2dde71659 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-6900000000-c5fee471922a8688dc98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-44af82827b749a0aec27 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029409 |
|---|
| FooDB ID | FDB000498 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055249 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5028066 |
|---|
| ChEBI ID | 85534 |
|---|
| PubChem Compound ID | 6560290 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|