| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:50:07 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023105 |
|---|
| Identification |
|---|
| Common Name | Sinapine |
|---|
| Class | Small Molecule |
|---|
| Description | An acylcholine in which the acyl group specified is sinapoyl. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
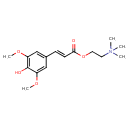
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(4-Hydroxy-3,5-dimethoxycinnamoyloxy)-N,N,N-trimethylethanaminium | ChEBI | | O-Sinapoylcholine | ChEBI | | Sinapoylcholine | ChEBI | | 2-[[3-(4-Hydroxy-3,5-dimethoxyphenyl)-1-oxo-2-propenyl]oxy]-N,N,N- trimethylethanaminium, 9ci | HMDB | | 4-Hydroxy-3,5-dimethoxycinnamate choline | HMDB | | Choline 4-hydroxy-3,5-dimethoxycinnamate, 8ci | HMDB | | Sinapine bisulfate | HMDB | | Sinapine bisulphate | HMDB | | Sugar phosphoric acid | HMDB | | trans-Sinapine | HMDB | | trans-Sinapoylcholine | HMDB |
|
|---|
| Chemical Formula | C16H24NO5 |
|---|
| Average Molecular Mass | 310.366 g/mol |
|---|
| Monoisotopic Mass | 310.165 g/mol |
|---|
| CAS Registry Number | 18696-26-9 |
|---|
| IUPAC Name | (2-{[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoyl]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | sinapine |
|---|
| SMILES | COC1=CC(\C=C/C(=O)OCC[N+](C)(C)C)=CC(OC)=C1O |
|---|
| InChI Identifier | InChI=1S/C16H23NO5/c1-17(2,3)8-9-22-15(18)7-6-12-10-13(20-4)16(19)14(11-12)21-5/h6-7,10-11H,8-9H2,1-5H3/p+1 |
|---|
| InChI Key | HUJXHFRXWWGYQH-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Cinnamic acid ester
- Methoxyphenol
- Dimethoxybenzene
- M-dimethoxybenzene
- Acyl choline
- Anisole
- Methoxybenzene
- Phenol ether
- Styrene
- Phenoxy compound
- Fatty acid ester
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Enoate ester
- Quaternary ammonium salt
- Alpha,beta-unsaturated carboxylic ester
- Tetraalkylammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Ether
- Organic oxide
- Organic salt
- Hydrocarbon derivative
- Amine
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Organopnictogen compound
- Organic cation
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9340000000-63c6b1f491d1742f581e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-07r9-9083000000-01fd82cd03199a2410f7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0ik9-0069000000-dae71e5c3cb5e8c98d01 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0090000000-689b623cad4015ad4305 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0kdi-0980000000-e85059fdfaf38b65fbf3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0092000000-57b6d7af206f0f8438e3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0090000000-c865ce6a2d45509c5636 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0090000000-c860008ae94305bdbb56 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0390000000-fe4aa244978824523259 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0kdj-0950000000-61508103ba9b618dbf8f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0090000000-bf3c90800cc09e89c513 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0090000000-e6a2d2e0e7afaaa89594 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0090000000-c17f367eb5b6d7c37692 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0090000000-4b0ecc4ed11995be1006 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029379 |
|---|
| FooDB ID | FDB004152 |
|---|
| Phenol Explorer ID | 557 |
|---|
| KNApSAcK ID | C00002777 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | O-SINAPOYLCHOLINE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sinapine |
|---|
| Chemspider ID | 80576 |
|---|
| ChEBI ID | 16353 |
|---|
| PubChem Compound ID | 89287 |
|---|
| Kegg Compound ID | C00933 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB23867 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|