| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:48:55 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023073 |
|---|
| Identification |
|---|
| Common Name | beta1-Tomatidine |
|---|
| Class | Small Molecule |
|---|
| Description | beta1-Tomatidine is found in garden tomato. beta1-Tomatidine is present in tomatoe |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
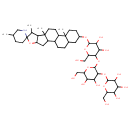
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b1-Tomatidine | Generator | | Β1-tomatidine | Generator | | b1-Tomatine | HMDB |
|
|---|
| Chemical Formula | C45H75NO17 |
|---|
| Average Molecular Mass | 902.074 g/mol |
|---|
| Monoisotopic Mass | 901.503 g/mol |
|---|
| CAS Registry Number | 17406-46-1 |
|---|
| IUPAC Name | 2-[(2-{[4,5-dihydroxy-2-(hydroxymethyl)-6-{5',7,9,13-tetramethyl-5-oxaspiro[pentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icosane-6,2'-piperidine]oxy}oxan-3-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl)oxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | 2-[(2-{[4,5-dihydroxy-2-(hydroxymethyl)-6-{5',7,9,13-tetramethyl-5-oxaspiro[pentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icosane-6,2'-piperidine]oxy}oxan-3-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl)oxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | CC1C2C(CC3C4CCC5CC(CCC5(C)C4CCC23C)OC2OC(CO)C(OC3OC(CO)C(O)C(O)C3OC3OC(CO)C(O)C(O)C3O)C(O)C2O)OC11CCC(C)CN1 |
|---|
| InChI Identifier | InChI=1S/C45H75NO17/c1-19-7-12-45(46-15-19)20(2)30-26(63-45)14-25-23-6-5-21-13-22(8-10-43(21,3)24(23)9-11-44(25,30)4)57-40-37(56)35(54)38(29(18-49)60-40)61-42-39(34(53)32(51)28(17-48)59-42)62-41-36(55)33(52)31(50)27(16-47)58-41/h19-42,46-56H,5-18H2,1-4H3 |
|---|
| InChI Key | DMUPZSDWJVULSC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroidal saponins. These are saponins in which the aglycone moiety is a steroid. The steroidal aglycone is usually a spirostane, furostane, spirosolane, solanidane, or curcubitacin derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroidal saponins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroidal saponin
- Diterpene glycoside
- Spirosolane skeleton
- Oligosaccharide
- Diterpenoid
- Steroidal alkaloid
- Azasteroid
- Terpene glycoside
- Glycosyl compound
- O-glycosyl compound
- Azaspirodecane
- Alkaloid or derivatives
- Oxane
- Piperidine
- Tetrahydrofuran
- Secondary alcohol
- Hemiaminal
- Polyol
- Secondary amine
- Acetal
- Secondary aliphatic amine
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Primary alcohol
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0331-0207890752-6bf3e02bdb1f3cd66c7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0215960300-e07f655f34e9526fd2e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02t9-1608940110-3d482991a1b643892b1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0imi-2613951745-f7ba5ec6ec6b30705e77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-2903840330-2f3fca562ebe6af8eed4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fr-4902820000-361b1f5d9d1f3810b1cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0004010049-e4b7644f529fe503da84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6w-0409210112-5ef4fab98f90276b65e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054k-7930800120-91bfaafb913c328665d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000119-82af0faca7e72eb22822 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-114i-7202330496-0e751c2a8b148caa2be6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054o-9311210200-70ea457e61802402a1fc | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029343 |
|---|
| FooDB ID | FDB000404 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 191536 |
|---|
| PubChem Compound ID | 76517846 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|