| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:48:53 UTC |
|---|
| Update Date | 2016-11-09 01:17:38 UTC |
|---|
| Accession Number | CHEM023072 |
|---|
| Identification |
|---|
| Common Name | Ceanothine E |
|---|
| Class | Small Molecule |
|---|
| Description | Ceanothine E is found in tea. Ceanothine E is an alkaloid from the root bark of Ceanothus americanus (New Jersey tea |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
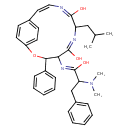
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-(Dimethylamino)-N-[7-(2-methylpropyl)-5,8-dioxo-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-10,12,14,15-tetraen-4-yl]benzenepropanamide, 9ci | HMDB | | N-[(10Z)-5,8-Dihydroxy-7-(2-methylpropyl)-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-phenylpropanimidate | HMDB |
|
|---|
| Chemical Formula | C34H40N4O4 |
|---|
| Average Molecular Mass | 568.706 g/mol |
|---|
| Monoisotopic Mass | 568.305 g/mol |
|---|
| CAS Registry Number | 23926-98-9 |
|---|
| IUPAC Name | (Z)-N-[(5E,8E,10Z)-5,8-dihydroxy-7-(2-methylpropyl)-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-phenylpropimidic acid |
|---|
| Traditional Name | (Z)-N-[(5E,8E,10Z)-5,8-dihydroxy-7-(2-methylpropyl)-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-phenylpropimidic acid |
|---|
| SMILES | CC(C)CC1\N=C(O)/C(\N=C(/O)C(CC2=CC=CC=C2)N(C)C)C(OC2=CC=C(C=C2)\C=C/N=C1/O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C34H40N4O4/c1-23(2)21-28-32(39)35-20-19-24-15-17-27(18-16-24)42-31(26-13-9-6-10-14-26)30(34(41)36-28)37-33(40)29(38(3)4)22-25-11-7-5-8-12-25/h5-20,23,28-31H,21-22H2,1-4H3,(H,35,39)(H,36,41)(H,37,40)/b20-19- |
|---|
| InChI Key | KCFAADIKGBVBFW-VXPUYCOJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Phenylalanine or derivatives
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Alkyl aryl ether
- Aralkylamine
- Fatty amide
- Fatty acyl
- Benzenoid
- Monocyclic benzene moiety
- Amino acid or derivatives
- Lactam
- Carboxamide group
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Oxacycle
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-2900000000-8e6cdce117b4526bd7ad | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0002-2900000000-87f483a8834873c4d500 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Ceanothine E,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014l-0307090000-6402467fbfd9821b29ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-0906000000-c5d44be79c385242bac2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pka-6903000000-23fab7f62e7ec769e14b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0101090000-a9e345593bb8b8247e12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05tf-1405090000-1bc86a17a05228a17cea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-5509200000-7af0b4ff0eebda30b00f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000090000-f9abb05dca2b272de274 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-2403290000-7a940754e2a9fc0d5f61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002o-9805110000-f6ea88e07ec591e5f101 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-c9d08b2fc36f0c85b507 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0209120000-12691cdec4f1f81481e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-2609500000-3ec778cf412f2f17c837 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029342 |
|---|
| FooDB ID | FDB000403 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055255 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4523141 |
|---|
| ChEBI ID | 176071 |
|---|
| PubChem Compound ID | 16058115 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|