| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:48:40 UTC |
|---|
| Update Date | 2016-11-09 01:17:37 UTC |
|---|
| Accession Number | CHEM023065 |
|---|
| Identification |
|---|
| Common Name | Mucronine D |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from the stem bark of Zizyphus jujuba (Chinese date) and Zizyphus jujuba variety inermis. Mucronine D is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
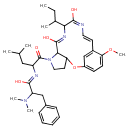
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Daechuine S9 | HMDB | | Mucronine a | HMDB | | N-{1-[(13E)-10-(butan-2-yl)-8,11-dihydroxy-16-methoxy-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-4-methyl-1-oxopentan-2-yl}-2-(dimethylamino)-3-phenylpropanimidate | Generator |
|
|---|
| Chemical Formula | C37H51N5O6 |
|---|
| Average Molecular Mass | 661.831 g/mol |
|---|
| Monoisotopic Mass | 661.384 g/mol |
|---|
| CAS Registry Number | 38496-00-3 |
|---|
| IUPAC Name | (Z)-N-{1-[(8Z,11E,13E)-10-(butan-2-yl)-8,11-dihydroxy-16-methoxy-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-4-methyl-1-oxopentan-2-yl}-2-(dimethylamino)-3-phenylpropimidic acid |
|---|
| Traditional Name | (Z)-N-{1-[(8Z,11E,13E)-8,11-dihydroxy-16-methoxy-10-(sec-butyl)-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-4-methyl-1-oxopentan-2-yl}-2-(dimethylamino)-3-phenylpropimidic acid |
|---|
| SMILES | CCC(C)C1NC(=O)C2C(CCN2C(=O)C(CC(C)C)NC(=O)C(CC2=CC=CC=C2)N(C)C)OC2=CC=C(OC)C(=C2)\C=C/NC1=O |
|---|
| InChI Identifier | InChI=1S/C37H51N5O6/c1-8-24(4)32-35(44)38-18-16-26-22-27(14-15-30(26)47-7)48-31-17-19-42(33(31)36(45)40-32)37(46)28(20-23(2)3)39-34(43)29(41(5)6)21-25-12-10-9-11-13-25/h9-16,18,22-24,28-29,31-33H,8,17,19-21H2,1-7H3,(H,38,44)(H,39,43)(H,40,45)/b18-16- |
|---|
| InChI Key | YTPWZBXRZAQHQB-VLGSPTGOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Phenylalanine or derivatives
- Leucine or derivatives
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- N-acylpyrrolidine
- Anisole
- Aralkylamine
- Alkyl aryl ether
- Fatty acyl
- Benzenoid
- Fatty amide
- Monocyclic benzene moiety
- Tertiary carboxylic acid amide
- Pyrrolidine
- Tertiary amine
- Carboxamide group
- Amino acid or derivatives
- Lactam
- Tertiary aliphatic amine
- Secondary carboxylic acid amide
- Azacycle
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic nitrogen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-3900001000-e7534b630b25ddcf8051 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dr-0212609000-ba1ed4c2e14488bb2a77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2900200000-ea270df8531ce01818df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-8902000000-64dd7879b58dc1ecf58e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0100009000-47e9a97e8053b3023ee6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06xx-2527419000-c2cc91feabba2df58168 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0601-3749502000-38ea25f504561c1dd579 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dr-0160409000-d4390c58dab2ef369787 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ik9-3591708000-2900c66bcc7f365e46fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9701000000-3cf66f6fce4d0f2027ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dr-0010509000-6cbfb554ec64d8d49c94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-1609302000-717f87ef3d798d9c6cb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-2629141000-c8d9a0aca4b343ccd280 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029335 |
|---|
| FooDB ID | FDB000396 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028609 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4523151 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5373023 |
|---|
| Kegg Compound ID | C10009 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|