| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:48:36 UTC |
|---|
| Update Date | 2016-11-09 01:17:37 UTC |
|---|
| Accession Number | CHEM023063 |
|---|
| Identification |
|---|
| Common Name | Amphibine H |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from the stem bark of Zizyphus jujuba (Chinese date). Amphibine H is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
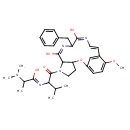
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(Dimethylamino)-N-[2-methyl-1-[[3,3a,12,13,14,15,16,16a-octahydro-8-methoxy-13,16-dioxo-14-(phenylmethyl)-5,9-metheno-9H-pyrrolo[3,2-b][1,5,8]oxadiazacyclopentadecin-1(2H)-yl]carbonyl]propyl]propanamide, 9ci | HMDB | | N-{1-[(13E)-10-benzyl-8,11-dihydroxy-16-methoxy-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-3-methyl-1-oxobutan-2-yl}-2-(dimethylamino)propanimidate | Generator |
|
|---|
| Chemical Formula | C33H43N5O6 |
|---|
| Average Molecular Mass | 605.724 g/mol |
|---|
| Monoisotopic Mass | 605.321 g/mol |
|---|
| CAS Registry Number | 52659-55-9 |
|---|
| IUPAC Name | (Z)-N-{1-[(8E,11E,13E)-10-benzyl-8,11-dihydroxy-16-methoxy-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-3-methyl-1-oxobutan-2-yl}-2-(dimethylamino)propimidic acid |
|---|
| Traditional Name | (Z)-N-{1-[(8E,11E,13E)-10-benzyl-8,11-dihydroxy-16-methoxy-2-oxa-6,9,12-triazatricyclo[13.3.1.0³,⁷]nonadeca-1(18),8,11,13,15(19),16-hexaen-6-yl]-3-methyl-1-oxobutan-2-yl}-2-(dimethylamino)propimidic acid |
|---|
| SMILES | COC1=CC=C2OC3CCN(C3\C(O)=N/C(CC3=CC=CC=C3)\C(O)=N/C=C/C1=C2)C(=O)C(\N=C(/O)C(C)N(C)C)C(C)C |
|---|
| InChI Identifier | InChI=1S/C33H43N5O6/c1-20(2)28(36-30(39)21(3)37(4)5)33(42)38-17-15-27-29(38)32(41)35-25(18-22-10-8-7-9-11-22)31(40)34-16-14-23-19-24(44-27)12-13-26(23)43-6/h7-14,16,19-21,25,27-29H,15,17-18H2,1-6H3,(H,34,40)(H,35,41)(H,36,39)/b16-14+ |
|---|
| InChI Key | KLYKBXVHBJWDJF-JQIJEIRASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9200100000-4c69cd89765418bd0060 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9200000000-e5f39b43a7cbf2b29527 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Amphibine H,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-9100354000-6fe75d9557e04f990224 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-6e053e297c007ecb099c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9310000000-7f2632e946dd705a6508 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0442449000-7229f43be3fd8e6e83f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pba-3504982000-ef7041619c605bf8e232 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9410320000-f50435cdd6bc453a3914 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0200029000-c0da49352491b7b5748b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-4201953000-1c5c2b5516c28ffd5bb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5109100000-509c70d8e9834950ed22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0100119000-5b6037a7a46e8f432089 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200555000-0bc44dc6c099f92b4cd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000200000-96e7bd4f04b1c73ccf56 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029333 |
|---|
| FooDB ID | FDB000394 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 51029223 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|