| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:48:07 UTC |
|---|
| Update Date | 2016-11-09 01:17:37 UTC |
|---|
| Accession Number | CHEM023048 |
|---|
| Identification |
|---|
| Common Name | L-Malic acid caffeate |
|---|
| Class | Small Molecule |
|---|
| Description | Caffeoylmalic acid is found in common bean. Caffeoylmalic acid is isolated from leaves of French bean (Phaseolus vulgaris) and from Trifolium pratense (red clover |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
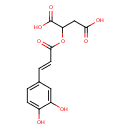
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Caffeoylmalate | Generator | | (S)-Phaselic acid | HMDB | | Phaseolic acid? | HMDB | | 2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}butanedioate | Generator | | Caffeoylmalic acid | MeSH | | L-Malate caffeate | Generator | | L-Malic acid caffeic acid | Generator |
|
|---|
| Chemical Formula | C13H12O8 |
|---|
| Average Molecular Mass | 296.230 g/mol |
|---|
| Monoisotopic Mass | 296.053 g/mol |
|---|
| CAS Registry Number | 53755-04-7 |
|---|
| IUPAC Name | 2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}butanedioic acid |
|---|
| Traditional Name | 2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}butanedioic acid |
|---|
| SMILES | OC(=O)CC(OC(=O)\C=C\C1=CC=C(O)C(O)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C13H12O8/c14-8-3-1-7(5-9(8)15)2-4-12(18)21-10(13(19)20)6-11(16)17/h1-5,10,14-15H,6H2,(H,16,17)(H,19,20)/b4-2+ |
|---|
| InChI Key | PMKQSEYPLQIEAY-DUXPYHPUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Cinnamic acid ester
- Tricarboxylic acid or derivatives
- Catechol
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Fatty acid ester
- Phenol
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-1910000000-74e3c09316c8d9fb3ea5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0avi-4146190000-5a765f56e5efd99345a3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0401-1790000000-ef531f4b3f8db4d508cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03xr-3940000000-ff40fcbd1f957b9e680e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-7900000000-187b75c221f4f3c49fff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ftb-1970000000-6b43ca38ba19571e6a67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bi-3920000000-98c6d2f5b48eb1a9ccc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03g0-3900000000-60d5ea04840a20f30f6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290000000-a44daa816456bebdbd8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9540000000-6be49add441a49df34b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3910000000-46381cd1c0d16863ebb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0920000000-a4c71d6efa8a850a8ec6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-ecf10995634e427e58a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-1900000000-ca91bf87f1f063f2269a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029318 |
|---|
| FooDB ID | FDB000379 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002765 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Caffeoylmalic acid |
|---|
| Chemspider ID | 4816367 |
|---|
| ChEBI ID | 493262 |
|---|
| PubChem Compound ID | 6124299 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|