| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:46:11 UTC |
|---|
| Update Date | 2016-11-09 01:17:37 UTC |
|---|
| Accession Number | CHEM022989 |
|---|
| Identification |
|---|
| Common Name | 3-Feruloyl-1,5-quinolactone |
|---|
| Class | Small Molecule |
|---|
| Description | 3-Feruloyl-1,5-quinolactone is a polyphenol compound found in foods of plant origin (PMID: 20428313) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
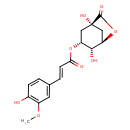
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Feruloyl-1,5-lactone | HMDB | | 3-Feruloyl-1,5-quinide | HMDB | | 3-Feruloylquinic acid lactone | HMDB | | (1R,3R,4R,5R)-1,4-Dihydroxy-7-oxo-6-oxabicyclo[3.2.1]octan-3-yl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C17H18O8 |
|---|
| Average Molecular Mass | 350.320 g/mol |
|---|
| Monoisotopic Mass | 350.100 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1R,3R,4R,5R)-1,4-dihydroxy-7-oxo-6-oxabicyclo[3.2.1]octan-3-yl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| Traditional Name | (1R,3R,4R,5R)-1,4-dihydroxy-7-oxo-6-oxabicyclo[3.2.1]octan-3-yl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| SMILES | COC1=C(O)C=CC(\C=C\C(=O)O[C@@H]2C[C@]3(O)C[C@@H](OC3=O)[C@@H]2O)=C1 |
|---|
| InChI Identifier | InChI=1S/C17H18O8/c1-23-11-6-9(2-4-10(11)18)3-5-14(19)24-12-7-17(22)8-13(15(12)20)25-16(17)21/h2-6,12-13,15,18,20,22H,7-8H2,1H3/b5-3+/t12-,13-,15-,17+/m1/s1 |
|---|
| InChI Key | KFVJGWDDYWUTHM-AWOKGZDASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Cinnamic acid ester
- Methoxyphenol
- Anisole
- Caprolactone
- Phenoxy compound
- Phenol ether
- Styrene
- Methoxybenzene
- Alkyl aryl ether
- Phenol
- Oxepane
- 1-hydroxy-2-unsubstituted benzenoid
- Fatty acid ester
- Benzenoid
- Monocyclic benzene moiety
- Fatty acyl
- Dicarboxylic acid or derivatives
- Gamma butyrolactone
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Tetrahydrofuran
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Organoheterocyclic compound
- Ether
- Oxacycle
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9523000000-c0e26d44c264d09a784f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-5231190000-e832b1719d0c623dc24f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ufr-0907000000-3a50d15344844119dc8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0901000000-e0cd2fa74d69ebf7502a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-1900000000-511a23220d0038d449f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0709000000-91470a394b4ed3c2dd96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05i0-0902000000-1864caad4d28bcf7a918 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1900000000-41e3fa516cfa36d0425d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0902000000-de5cde31b78169166170 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0901000000-944ad5db00b310d0be94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dj-1903000000-be76a87397666ad6d2dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0309000000-03e64a36ba0a98b4212c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-0902000000-257092d95b68ede4d495 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-1900000000-0e16c6c8dded3f2faaa8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029289 |
|---|
| FooDB ID | FDB000293 |
|---|
| Phenol Explorer ID | 544 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776759 |
|---|
| ChEBI ID | 175470 |
|---|
| PubChem Compound ID | 102210470 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|