| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:46:00 UTC |
|---|
| Update Date | 2016-11-09 01:17:37 UTC |
|---|
| Accession Number | CHEM022981 |
|---|
| Identification |
|---|
| Common Name | Avenanthramide 2c |
|---|
| Class | Small Molecule |
|---|
| Description | N-(3,4-Dihydroxycinnamoyl)-2-amino-5-hydroxybenzoic acid is found in cereals and cereal products. N-(3,4-Dihydroxycinnamoyl)-2-amino-5-hydroxybenzoic acid is isolated from oats (Avena sativa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
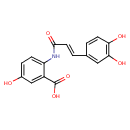
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-(3,4-Dihydroxycinnamoyl)-5-hydroxyanthranilic acid | HMDB | | Avenanthramide | MeSH, HMDB | | Avenanthramide 2C | MeSH, HMDB | | Avenanthramide-c | MeSH, HMDB | | N-(3',4'-Dihydroxycinnamoyl)-5-hydroxyanthranilic acid | MeSH, HMDB | | Avenanthramide-2C | MeSH, HMDB | | 2-{[(2E)-3-(3,4-dihydroxyphenyl)-1-hydroxyprop-2-en-1-ylidene]amino}-5-hydroxybenzoate | Generator |
|
|---|
| Chemical Formula | C16H13NO6 |
|---|
| Average Molecular Mass | 315.278 g/mol |
|---|
| Monoisotopic Mass | 315.074 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enamido]-5-hydroxybenzoic acid |
|---|
| Traditional Name | 2-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enamido]-5-hydroxybenzoic acid |
|---|
| SMILES | OC(=O)C1=CC(O)=CC=C1NC(=O)\C=C\C1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H13NO6/c18-10-3-4-12(11(8-10)16(22)23)17-15(21)6-2-9-1-5-13(19)14(20)7-9/h1-8,18-20H,(H,17,21)(H,22,23)/b6-2+ |
|---|
| InChI Key | IDUUXROOZBOOPH-QHHAFSJGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as avenanthramides. These are a group of phenolic alkaloids consisting of conjugate of three phenylpropanoids (ferulic, caffeic, or p-coumaric acid) and anthranilic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Avenanthramides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Avenanthramide
- N-cinnamoylanthranilic acid
- Acylaminobenzoic acid or derivatives
- Cinnamic acid amide
- Hydroxybenzoic acid
- Anilide
- Benzoic acid or derivatives
- Benzoic acid
- Styrene
- N-arylamide
- Catechol
- Benzoyl
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous amide
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kk-0961000000-44dbc62c555f06b47f7f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-000i-1001090000-53a821e1e639ff675118 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0944000000-5e5598cbbeecb8348ac7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0920000000-1b9e5ede80b19281ed06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-3900000000-46e41c6f4643f73a7a96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03k9-0296000000-14b2323a1e6e5ff7cbad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0h90-0691000000-8b8e4683c02f3c041f74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1900000000-314538513f94732d5b12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0932000000-5d3591a255d5d5cbfa17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-0940000000-0f1360efb2b43e67087f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ks-0910000000-1e8610c76ae0ea403063 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0392000000-43f87b14905d03692265 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0920000000-5e17ae5c4582f4714289 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0543-2920000000-c1737d3db456bb532dfc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038576 |
|---|
| FooDB ID | FDB000284 |
|---|
| Phenol Explorer ID | 533 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9897916 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11723200 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|