| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:45:45 UTC |
|---|
| Update Date | 2016-11-09 01:17:36 UTC |
|---|
| Accession Number | CHEM022971 |
|---|
| Identification |
|---|
| Common Name | 5-8'-Benzofuran dehydrodiferulic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
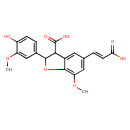
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-[(1E)-2-Carboxyeth-1-en-1-yl]-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydro-1-benzofuran-3-carboxylate | Generator | | 5-8'-Benzofuran dehydrodiferulate | Generator |
|
|---|
| Chemical Formula | C20H18O8 |
|---|
| Average Molecular Mass | 386.352 g/mol |
|---|
| Monoisotopic Mass | 386.100 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-[(1E)-2-carboxyeth-1-en-1-yl]-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydro-1-benzofuran-3-carboxylic acid |
|---|
| Traditional Name | 5-[(1E)-2-carboxyeth-1-en-1-yl]-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydro-1-benzofuran-3-carboxylic acid |
|---|
| SMILES | COC1=C2OC(C(C(O)=O)C2=CC(\C=C\C(O)=O)=C1)C1=CC=C(O)C(OC)=C1 |
|---|
| InChI Identifier | InChI=1S/C20H18O8/c1-26-14-9-11(4-5-13(14)21)18-17(20(24)25)12-7-10(3-6-16(22)23)8-15(27-2)19(12)28-18/h3-9,17-18,21H,1-2H3,(H,22,23)(H,24,25)/b6-3+ |
|---|
| InChI Key | JTHPLBUVRLOJBB-ZZXKWVIFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-arylbenzofuran flavonoids. These are phenylpropanoids containing the 2-phenylbenzofuran moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | 2-arylbenzofuran flavonoids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | 2-arylbenzofuran flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arylbenzofuran flavonoid
- Neolignan skeleton
- Coumaric acid or derivatives
- Methoxyphenol
- Benzofuran
- Coumaran
- Phenoxy compound
- Methoxybenzene
- Anisole
- Styrene
- Phenol ether
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Ether
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00ku-0109000000-ebc8f56ea1c1ea84d36d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kg-0029000000-3664ea98e1815401683f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ukd-0920000000-b1f0bf36cd27a24b9185 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-0009000000-22057ca24a2919b8f06b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-0019000000-1f31f869368215e10ac5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0649000000-0e1a8db10bee85209b6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-0009000000-edc4bb1c9270b9af3368 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00rj-0159000000-56deaf2703d1becd549f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002n-0359000000-59156418a7198905f74b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0097000000-84f5023464256f7dc5e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-79dcced1c59f1aa064ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-0469000000-6b0bfcf7f57f4ab31be1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0301724 |
|---|
| FooDB ID | FDB000273 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4800495 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6079067 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|