| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:45:44 UTC |
|---|
| Update Date | 2016-11-09 01:17:36 UTC |
|---|
| Accession Number | CHEM022970 |
|---|
| Identification |
|---|
| Common Name | 8-8'-Dehydrodiferulic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 8-8'-Dehydrodiferulic acid is a polyphenol compound found in foods of plant origin (PMID: 20428313) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
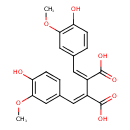
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8-8'-Dehydrodiferulate | Generator | | (2E)-Bis[(4-hydroxy-3-methoxyphenyl)methylidene]butanedioate | HMDB |
|
|---|
| Chemical Formula | C20H18O8 |
|---|
| Average Molecular Mass | 386.352 g/mol |
|---|
| Monoisotopic Mass | 386.100 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2Z,3E)-bis[(4-hydroxy-3-methoxyphenyl)methylidene]butanedioic acid |
|---|
| Traditional Name | (2Z,3E)-bis[(4-hydroxy-3-methoxyphenyl)methylidene]butanedioic acid |
|---|
| SMILES | COC1=C(O)C=CC(\C=C(\C(O)=O)/C(=C/C2=CC(OC)=C(O)C=C2)/C(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C20H18O8/c1-27-17-9-11(3-5-15(17)21)7-13(19(23)24)14(20(25)26)8-12-4-6-16(22)18(10-12)28-2/h3-10,21-22H,1-2H3,(H,23,24)(H,25,26)/b13-7-,14-8+ |
|---|
| InChI Key | SPKZMOSDXTYXLK-MFUUIURDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignans, neolignans and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Norlignan skeleton
- Cinnamic acid
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Hydroxycinnamic acid or derivatives
- Hydroxycinnamic acid
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Ether
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ko-0409000000-73deae33c335e184e345 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0bt9-3000029000-fffb0377a23ea253dd4e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0109000000-9c7609ea8cc607bde9d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ko-0229000000-2925758ef7b4b0f5d02c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052k-0694000000-2f7413d682542540c85d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-d891ff7c770d199a2495 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000l-0329000000-46cba6d908bc2a00a765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-0904000000-16d0f7babfe6c40538db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-0059000000-2afdeabfedfeb460ca2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0049000000-f517195a4935f779254d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-0169000000-923f6f6b34faa93da1f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0209000000-db1ab7cae585ebe0c389 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0adi-1719000000-8f6f8555e16bde401fa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2879000000-51984780b51828710be4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029277 |
|---|
| FooDB ID | FDB000272 |
|---|
| Phenol Explorer ID | 517 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 29336462 |
|---|
| ChEBI ID | 175914 |
|---|
| PubChem Compound ID | 131750840 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|