| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 20:44:50 UTC |
|---|
| Update Date | 2016-11-09 01:17:36 UTC |
|---|
| Accession Number | CHEM022942 |
|---|
| Identification |
|---|
| Common Name | Sanguisorbic acid dilactone |
|---|
| Class | Small Molecule |
|---|
| Description | A polyphenol compound found in foods of plant origin (PhenolExplorer). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
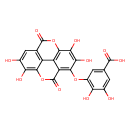
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sanguisorbate dilactone | Generator | | 3,4-Dihydroxy-5-({6,7,13,14-tetrahydroxy-3,10-dioxo-2,9-dioxatetracyclo[6.6.2.0⁴,¹⁶.0¹¹,¹⁵]hexadeca-1(15),4,6,8(16),11,13-hexaen-5-yl}oxy)benzoate | Generator |
|
|---|
| Chemical Formula | C21H10O13 |
|---|
| Average Molecular Mass | 470.296 g/mol |
|---|
| Monoisotopic Mass | 470.012 g/mol |
|---|
| CAS Registry Number | 82203-11-0 |
|---|
| IUPAC Name | 3,4-dihydroxy-5-({6,7,13,14-tetrahydroxy-3,10-dioxo-2,9-dioxatetracyclo[6.6.2.0⁴,¹⁶.0¹¹,¹⁵]hexadeca-1(14),4,6,8(16),11(15),12-hexaen-5-yl}oxy)benzoic acid |
|---|
| Traditional Name | 3,4-dihydroxy-5-({6,7,13,14-tetrahydroxy-3,10-dioxo-2,9-dioxatetracyclo[6.6.2.0⁴,¹⁶.0¹¹,¹⁵]hexadeca-1(14),4,6,8(16),11(15),12-hexaen-5-yl}oxy)benzoic acid |
|---|
| SMILES | OC(=O)C1=CC(OC2=C3C(=O)OC4=C(O)C(O)=CC5=C4C3=C(OC5=O)C(O)=C2O)=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H10O13/c22-6-1-4(19(28)29)2-8(12(6)24)32-18-11-10-9-5(20(30)33-17(10)14(26)15(18)27)3-7(23)13(25)16(9)34-21(11)31/h1-3,22-27H,(H,28,29) |
|---|
| InChI Key | DBLLWHYTKRSJEV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydrolyzable tannins. These are tannins with a structure characterized by either of the following models. In model 1, the structure contains galloyl units (in some cases, shikimic acid units) that are linked to diverse polyol carbohydrate-, catechin-, or triterpenoid units. In model 2, contains at least two galloyl units C-C coupled to each other, and do not contain a glycosidically linked catechin unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tannins |
|---|
| Sub Class | Hydrolyzable tannins |
|---|
| Direct Parent | Hydrolyzable tannins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrolyzable tannin
- Ellagic_acid
- 7,8-dihydroxycoumarin
- Gallic acid or derivatives
- Dihydroxybenzoic acid
- Isocoumarin
- Diaryl ether
- Coumarin
- Hydroxybenzoic acid
- Benzopyran
- 1-benzopyran
- 2-benzopyran
- Benzoic acid
- Benzoic acid or derivatives
- Phenoxy compound
- Phenol ether
- Catechol
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- Pyranone
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Pyran
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Vinylogous ester
- Lactone
- Monocarboxylic acid or derivatives
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Ether
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f96-0001900000-30f35ff87b3ab59b0dcb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-2000049000-22e6acf9266b9fc91a08 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_17) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_18) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_15) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_26) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Sanguisorbic acid dilactone,3TMS,#10" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-5684b40d6a63f93265ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0umi-0101900000-efba53849560a720a54c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfs-1957200000-8039ee0bfd80ee9ba578 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001900000-2a81ee19e7844b62df57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-0312900000-136c7e8eea2f61266a4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0891000000-c9a6f03c6472a9014153 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0100900000-b537e9971b259dfd21eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0100900000-0a9aaadcbc2b7180377f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f96-2509800000-6a3dbbc84ebbb60aea0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kb-0070900000-9a8f8eaee9989ee069df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00vi-0904800000-415121812e857c4d0f9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ke-1119400000-a18d65a9dd1b4174a957 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029274 |
|---|
| FooDB ID | FDB000232 |
|---|
| Phenol Explorer ID | 443 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 15934028 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|