| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:54:10 UTC |
|---|
| Update Date | 2016-11-09 01:17:35 UTC |
|---|
| Accession Number | CHEM022803 |
|---|
| Identification |
|---|
| Common Name | Quinic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Quinic acid is a sugar acid. It is also a cyclitol, a cyclic polyol. More specifically, quinic acid is a crystalline acid obtained from cinchona bark, coffee beans, tobacco leaves, carrot leaves, apples, peaches, pears, plums, vegetables etc. Quinic acid can also be made synthetically by hydrolysis of chlorogenic acid. Quinic acid is implicated in the perceived acidity of coffee. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Feces
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
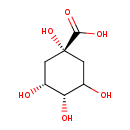
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Quinate | Generator | | (1R,3R,4S)-1,3,4,5-Tetrahydroxycyclohexane-1-carboxylate | HMDB | | (-)-Quinate | HMDB | | (-)-Quinic acid | HMDB | | Chinate | HMDB | | Chinic acid | HMDB | | D-Quinic acid | HMDB | | Kinate | HMDB | | Kinic acid | HMDB | | L-Quinate | HMDB | | L-Quinic acid | HMDB |
|
|---|
| Chemical Formula | C7H12O6 |
|---|
| Average Molecular Mass | 192.167 g/mol |
|---|
| Monoisotopic Mass | 192.063 g/mol |
|---|
| CAS Registry Number | 77-95-2 |
|---|
| IUPAC Name | (1R,3R,4S)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid |
|---|
| Traditional Name | (1R,3R,4S)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid |
|---|
| SMILES | OC1C[C@@](O)(C[C@@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4?,5-,7+/m1/s1 |
|---|
| InChI Key | AAWZDTNXLSGCEK-RKGSPJAZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinic acids and derivatives. Quinic acids and derivatives are compounds containing a quinic acid moiety (or a derivative thereof), which is a cyclitol made up of a cyclohexane ring that bears four hydroxyl groups at positions 1,3.4, and 5, as well as a carboxylic acid at position 1. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Quinic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinic acid
- Cyclohexanol
- Hydroxy acid
- Alpha-hydroxy acid
- Tertiary alcohol
- Secondary alcohol
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-9800000000-fe303752b78b547d4e54 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-000i-5101950000-4c2d01cb772e2291e41a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002g-0900000000-59471dc3f2bf86989e30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0900000000-d0b09550df3fa08a1420 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-5900000000-ab505f7c0b75a85ee3cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-0900000000-fe8d862acce93dd7eb84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-3900000000-f7e8be1495cce9a344ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05bb-9700000000-e16830e0be835671623b | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003072 |
|---|
| FooDB ID | FDB001170 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001201 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | QUINATE |
|---|
| METLIN ID | 3329 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Quinic_acid |
|---|
| Chemspider ID | 16498843 |
|---|
| ChEBI ID | 17521 |
|---|
| PubChem Compound ID | 6508 |
|---|
| Kegg Compound ID | C00296 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Nagai, Naoshi; Kuboyama, Hisaharu; Enya, Masahiro. Method for preparation of quinic acid and its esters. Jpn. Kokai Tokkyo Koho (2000), 7 pp. | | 2. Wittemer SM, Ploch M, Windeck T, Muller SC, Drewelow B, Derendorf H, Veit M: Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine. 2005 Jan;12(1-2):28-38. | | 3. Akesson C, Lindgren H, Pero RW, Leanderson T, Ivars F: Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100. Int Immunopharmacol. 2005 Jan;5(1):219-29. |
|
|---|