| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:53:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:34 UTC |
|---|
| Accession Number | CHEM022788 |
|---|
| Identification |
|---|
| Common Name | alpha-Copaene |
|---|
| Class | Small Molecule |
|---|
| Description | A naphthalenesulfonic acid that is naphthalene-1-sulfonic acid substituted by a phenylamino group at position 8. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Feces
|
|---|
| Contaminant Type | Not Available |
|---|
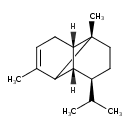
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(Phenylamino)-8-naphthalenesulfonic acid | ChEBI | | 1-ANILINO-8-naphthalene sulfonATE | ChEBI | | 1-Anilino-8-naphthalenesulfonate | ChEBI | | 1-Anilino-8-naphthalenesulfonic acid | ChEBI | | 8-Anilino-1-naphthalene sulfonic acid | ChEBI | | 8-Anilinonaphthalene-1-sulphonic acid | ChEBI | | ANS | ChEBI | | 1-(Phenylamino)-8-naphthalenesulfonate | Generator | | 1-(Phenylamino)-8-naphthalenesulphonate | Generator | | 1-(Phenylamino)-8-naphthalenesulphonic acid | Generator | | 1-ANILINO-8-naphthalene sulfonic acid | Generator | | 1-ANILINO-8-naphthalene sulphonate | Generator | | 1-ANILINO-8-naphthalene sulphonic acid | Generator | | 1-Anilino-8-naphthalenesulphonate | Generator | | 1-Anilino-8-naphthalenesulphonic acid | Generator | | 8-Anilino-1-naphthalene sulphonate | Generator | | 8-Anilino-1-naphthalene sulphonic acid | Generator | | 8-Anilinonaphthalene-1-sulfonate | Generator | | 8-Anilinonaphthalene-1-sulfonic acid | Generator | | 8-Anilinonaphthalene-1-sulphonate | Generator | | 1-Anilino-8-naphthalenesulfonate, monoammonium salt, hemihydrate | HMDB | | 1-Anilino-8-naphthalenesulfonate, monosodium salt | HMDB | | 1-Anilinonaphthalene-8-sulfonic acid | HMDB | | 1-Anilino-8-naphthalenesulfonate, magnesium (2:1) | HMDB | | 1,8-ANS | HMDB | | 1-Anilino-8-naphthalenesulfonate, 3H-labeled | HMDB | | 1-Anilino-8-naphthalenesulfonate, ion(1-) | HMDB | | 1-Anilino-8-naphthalenesulfonate, monoammonium salt | HMDB | | (1S,6S,7S,8S)-8-Isopropyl-1,3-dimethyltricyclo[4.4.0.0(2,7)]dec-3-ene | ChEBI | | 8-Isopropyl-1,3-dimethyltricyclo(4.4.0.02,7)dec-3-ene | ChEBI | | Copaene | ChEBI | | a-Copaene | Generator | | α-copaene | Generator |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.357 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 3856-25-5 |
|---|
| IUPAC Name | (1S,6S,7S,8S)-1,3-dimethyl-8-(propan-2-yl)tricyclo[4.4.0.0^{2,7}]dec-3-ene |
|---|
| Traditional Name | (1S,6S,7S,8S)-8-isopropyl-1,3-dimethyltricyclo[4.4.0.0^{2,7}]dec-3-ene |
|---|
| SMILES | [H][C@@]12C3C(C)=CC[C@]1([H])[C@]3(C)CC[C@H]2C(C)C |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-9(2)11-7-8-15(4)12-6-5-10(3)14(15)13(11)12/h5,9,11-14H,6-8H2,1-4H3/t11-,12-,13-,14?,15-/m0/s1 |
|---|
| InChI Key | VLXDPFLIRFYIME-XIQJJJERSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-naphthalene sulfonates. These are organic aromatic compounds that contain a naphthalene moiety that carries a sulfonic acid group at the 1-position. Naphthalene is a bicyclic compound that is made up of two fused benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Naphthalene sulfonic acids and derivatives |
|---|
| Direct Parent | 1-naphthalene sulfonates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-naphthalene sulfonate
- 1-naphthalene sulfonic acid or derivatives
- Arylsulfonic acid or derivatives
- 1-sulfo,2-unsubstituted aromatic compound
- Aniline or substituted anilines
- Monocyclic benzene moiety
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Sulfonyl
- Organosulfonic acid
- Secondary amine
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organosulfur compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03dl-6910000000-819571f4000f4ce2f677 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-a9c75307ad9fa7e5726b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0390000000-12dc458834cb96e2536d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059i-0920000000-4d0945c6547417b0848d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-5fefaad65bd188e67a4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-2342a92a86b61876ef46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0950000000-518835a78468ff13b3a1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04474 |
|---|
| HMDB ID | HMDB0061854 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 8-Anilinonaphthalene-1-sulfonic_acid |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 39708 |
|---|
| PubChem Compound ID | 1369 |
|---|
| Kegg Compound ID | C11326 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=14645081 | | 2. Lewin M, Saccomani G, Schackmann R, Sachs G: Use of 1-anilino-8-naphthalene-sulfonate as a probe of gastric vesicle transport. J Membr Biol. 1977 Apr 22;32(3-4):301-18. | | 3. Stiborova M, Lapka R, Leblova S: The bonding of 8-anilino-1-naphthalene sulfonate to rape (Brassica napus) alcohol dehydrogenase. FEBS Lett. 1979 Aug 15;104(2):309-12. | | 4. Mantsala P, Lang M: 1 Anilino-8-naphthalene sulfonate and n-phenyl-1-naphthylamine as the indicators of bacterial thermosensitivity. FEBS Lett. 1973 Nov 1;36(3):265-7. | | 5. Vanderkooi J, Martonosi A: Sarcoplasmic reticulum. 8. Use of 8-anilino-1-naphthalene sulfonate as conformational probe on biological membranes. Arch Biochem Biophys. 1969 Aug;133(1):153-63. | | 6. Vanderkooi JM, Martonosi A: Sarcoplasmic reticulum. XII. The interaction of 8-anilino-1-naphthalene sulfonate with skeletal muscle microsomes. Arch Biochem Biophys. 1971 May;144(1):87-98. | | 7. Nakatani H, Haga M, Hiromi K: Kinetic studies on binding of bovine serum albumin with 1-anilino-8-naphthalene sulfonate. FEBS Lett. 1974 Aug 1;43(3):293-6. | | 8. Nagradova NK, Asryants RA, Ivanov MV: Interaction of 1-anilino-8-naphthalene sulfonate with yeast glyceraldehyde-3-phosphate dehydrogenase. Experientia. 1971 Oct 15;27(10):1169-70. | | 9. Nerli B, Pico G: Influence of the medium conditions on the 1-anilino-8-naphthalene sulfonate-bovine serum albumin binding. Arch Int Physiol Biochim Biophys. 1994 Jan-Feb;102(1):5-8. | | 10. Gabellieri E, Strambini GB: Perturbation of protein tertiary structure in frozen solutions revealed by 1-anilino-8-naphthalene sulfonate fluorescence. Biophys J. 2003 Nov;85(5):3214-20. |
|
|---|