| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:52:59 UTC |
|---|
| Update Date | 2016-11-09 01:17:34 UTC |
|---|
| Accession Number | CHEM022787 |
|---|
| Identification |
|---|
| Common Name | (±)-cis-Linalyl oxide |
|---|
| Class | Small Molecule |
|---|
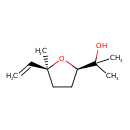
| Description | A monoterpenoid that is 2-(2-hydroxypropan-2-yl)oxolane carying additional methyl and vinyl substituents both located at position 5 (the 2R,5S-diastereomer). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Feces
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| cis-Linalyl oxide | ChEBI | | (Z)-Furanoid linalool oxide | HMDB | | 5-ethenyltetrahydro-a,a,5-Trimethyl-(2R,5S)-rel-2-furanmethanol | HMDB | | 5-ethenyltetrahydro-a,a,5-Trimethyl-cis-2-furanmethanol | HMDB | | cis-Furanoid linalool oxide | HMDB | | cis-Linalool 3,6-oxide | HMDB | | Linalool oxide b | HMDB | | Linalool oxide I | HMDB |
|
|---|
| Chemical Formula | C10H18O2 |
|---|
| Average Molecular Mass | 170.249 g/mol |
|---|
| Monoisotopic Mass | 170.131 g/mol |
|---|
| CAS Registry Number | 5989-33-3 |
|---|
| IUPAC Name | 2-[(2R,5S)-5-ethenyl-5-methyloxolan-2-yl]propan-2-ol |
|---|
| Traditional Name | 2-[(2R,5S)-5-ethenyl-5-methyloxolan-2-yl]propan-2-ol |
|---|
| SMILES | CC(C)(O)[C@H]1CC[C@](C)(O1)C=C |
|---|
| InChI Identifier | InChI=1S/C10H18O2/c1-5-10(4)7-6-8(12-10)9(2,3)11/h5,8,11H,1,6-7H2,2-4H3/t8-,10-/m1/s1 |
|---|
| InChI Key | BRHDDEIRQPDPMG-PSASIEDQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofurans. These are heterocyclic compounds containing a saturated, aliphatic, five-membered ring where a carbon is replaced by an oxygen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydrofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydrofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofuran
- Tertiary alcohol
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9100000000-d76b0d8bd5839f5bad93 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-003r-9720000000-d7880141bef77c136d15 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1900000000-3717374e13c0e4e46e01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fl0-7900000000-1a57b949dcbd663fc1eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pbi-9000000000-29f24016ec67ee4e749b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-92d23807ba882368b9c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-9591ca26dd3aa91ec77b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00m0-9400000000-adb5d889f7dfc0e0d558 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-8900000000-a536912550058a32831e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7p-9100000000-b3759eefd18d5faed102 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kc6-9000000000-369ce7815f6cf7814da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-efbd93a1a83feefc94df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-3900000000-6b8e798821e4115bf1d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-ace0a44f890d3bb40734 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040726 |
|---|
| FooDB ID | FDB020534 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034746 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4933975 |
|---|
| ChEBI ID | 132839 |
|---|
| PubChem Compound ID | 6428573 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01808 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|