| Synonyms | | Value | Source |
|---|
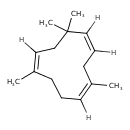
| (1E,4E,8E)-2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene | HMDB | | (1E,4E,8E)-2,6,6,9-Tetramethylcycloundeca-1,4,8-triene | HMDB | | (1E,4E,8E)-alpha-Humulene | HMDB | | (1E,4E,8E)-Humula-1(11),4,8-triene | HMDB | | (e,e,e)-2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene | HMDB | | 2,6,6,9-Tetramethyl-(1E,4E,8E)-1,4,8-cycloundecatriene | HMDB | | 2,6,6,9-Tetramethyl-(e,e,e)-1,4,8-cycloundecatriene | HMDB | | 2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene | HMDB | | 2,6,6,9-Tetramethyl-cycloundeca-1,4,8-triene | HMDB | | 3,7,10-Humulatriene | HMDB | | a-Caryophyllene (obsol.) | HMDB | | a-Humulene | HMDB, Generator | | alpha -Humulen | HMDB | | alpha -Humulene | HMDB | | alpha -Humulene (alpha -caryophyllene) | HMDB | | alpha -Humulenen | HMDB | | alpha-Caryophyllene | HMDB | | Humulene | HMDB | | alpha-Humulene | MeSH | | Α-humulene | Generator |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-Q (Non-derivatized) | splash10-0006-9300000000-96e2ddad1a99d808b4dd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0920000000-3e5845e9275b1b3231fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-d1375940e9b7ed7922b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2930000000-cdb29a58477970796a70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-4900000000-788e8bfc0021846819ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-8ecbabe7146ad2762752 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0390000000-984bd28446d79854bd71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ri-1900000000-139cbaab33ea7f90de6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-0920000000-90d0407fa9aa4598cee3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-c084f3d998cc56a199d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0190000000-0612dd5822c06422c9bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0079-0900000000-358d2a7dc14a6841b1df | Spectrum |
|
|---|