| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:51:09 UTC |
|---|
| Update Date | 2016-11-09 01:17:34 UTC |
|---|
| Accession Number | CHEM022761 |
|---|
| Identification |
|---|
| Common Name | Chenodeoxycholic acid 3-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Chenodeoxycholic acid 3-sulfate is produced by the action of enzymes existing in the microbial flora of the colonic environment. The major phase II metabolism of bile acids is sulfation mainly catalyzed by the hydroxysteroid sulfotransferase, forming the 3-sulfate conjugate. (PMID: 16949895). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Feces
|
|---|
| Contaminant Type | Not Available |
|---|
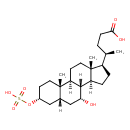
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Chenodeoxycholate 3-sulfate | Generator | | Chenodeoxycholate 3-sulphate | Generator | | Chenodeoxycholic acid 3-sulfuric acid | Generator | | Chenodeoxycholic acid 3-sulphuric acid | Generator | | Chenodeoxycholate sulfate conjugate | MeSH | | Chenodeoxycholic acid sulfate conjugate | MeSH | | CDCS | MeSH | | 3-Sulfochenodeoxycholate | HMDB | | 3-Sulfochenodeoxycholic acid | HMDB | | Chenodeoxycholic acid 3-sulphate | HMDB | | Chenodeoxycholic acid 3a-sulfate | HMDB | | Chenodeoxycholic acid 3a-sulphate | HMDB |
|
|---|
| Chemical Formula | C24H40O7S |
|---|
| Average Molecular Mass | 472.635 g/mol |
|---|
| Monoisotopic Mass | 472.249 g/mol |
|---|
| CAS Registry Number | 59132-32-0 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,5R,7R,9R,10R,11S,14R,15R)-9-hydroxy-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2S,5R,7R,9R,10R,11S,14R,15R)-9-hydroxy-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCC(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)C[C@]2([H])C[C@@H](CC[C@]12C)OS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C24H40O7S/c1-14(4-7-21(26)27)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(31-32(28,29)30)12-15(23)13-20(22)25/h14-20,22,25H,4-13H2,1-3H3,(H,26,27)(H,28,29,30)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 |
|---|
| InChI Key | WHMOBEGYTDWMIG-BSWAIDMHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monohydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or any of their derivatives bearing a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Monohydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monohydroxy bile acid, alcohol, or derivatives
- Sulfated steroid skeleton
- Hydroxysteroid
- 7-hydroxysteroid
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Cyclic alcohol
- Organic sulfuric acid or derivatives
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0536-0667900000-f5e817c86afceff3bc92 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0udi-4221349000-0993c416b847dc9363a2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0002900000-abbbbfd5950c6cf2e226 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0009200000-087c5d2f48d7160b5dbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-1229200000-7f1303ebc6e2d806539e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0001900000-bff4b187cc87bfca1cd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-2009400000-eab51a83a4551fd439fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5a-9007100000-7432e93f953dc907a065 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-29034312137a9db3fb88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1000900000-ade2350b3441a8a2d002 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9006400000-0a3dcf516ded28a9a1aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0002900000-06cd8122555b78f76406 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1019200000-1a2383b307a87525bad2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-1589000000-3a790fd1e8a788615b2e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002586 |
|---|
| FooDB ID | FDB023031 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6719 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 13628372 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 21252312 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Goto T, Myint KT, Sato K, Wada O, Kakiyama G, Iida T, Hishinuma T, Mano N, Goto J: LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Feb 1;846(1-2):69-77. Epub 2006 Sep 1. | | 2. Robben J, Janssen G, Merckx R, Eyssen H: Formation of delta 2- and delta 3-cholenoic acids from bile acid 3-sulfates by a human intestinal Fusobacterium strain. Appl Environ Microbiol. 1989 Nov;55(11):2954-9. |
|
|---|