| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:43:40 UTC |
|---|
| Update Date | 2016-11-09 01:17:33 UTC |
|---|
| Accession Number | CHEM022634 |
|---|
| Identification |
|---|
| Common Name | desmethylclomipramine |
|---|
| Class | Small Molecule |
|---|
| Description | desmethylclomipramine is a metabolite of clomipramine. Clomipramine (trademarked as Anafranil) is a tricyclic antidepressant (TCA). It was developed in the 1960s by the Swiss drug manufacturer Geigy (now known as Novartis) and has been in clinical use worldwide ever since. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
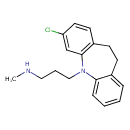
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Demethylclomipramine | HMDB | | Desmethylclomipramine hydrochloride | HMDB | | Demethylchlorimipramine | HMDB | | Desmethylchlorimipramine | HMDB |
|
|---|
| Chemical Formula | C18H21ClN2 |
|---|
| Average Molecular Mass | 300.826 g/mol |
|---|
| Monoisotopic Mass | 300.139 g/mol |
|---|
| CAS Registry Number | 303-48-0 |
|---|
| IUPAC Name | (3-{14-chloro-2-azatricyclo[9.4.0.0³,⁸]pentadeca-1(11),3,5,7,12,14-hexaen-2-yl}propyl)(methyl)amine |
|---|
| Traditional Name | (3-{14-chloro-2-azatricyclo[9.4.0.0³,⁸]pentadeca-1(11),3,5,7,12,14-hexaen-2-yl}propyl)(methyl)amine |
|---|
| SMILES | CNCCCN1C2=CC=CC=C2CCC2=C1C=C(Cl)C=C2 |
|---|
| InChI Identifier | InChI=1S/C18H21ClN2/c1-20-11-4-12-21-17-6-3-2-5-14(17)7-8-15-9-10-16(19)13-18(15)21/h2-3,5-6,9-10,13,20H,4,7-8,11-12H2,1H3 |
|---|
| InChI Key | VPIXQGUBUKFLRF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzazepines. Dibenzazepines are compounds with two benzene rings connected by an azepine ring. Azepine is an unsaturated seven-member heterocycle with one nitrogen atom replacing a carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzazepines |
|---|
| Sub Class | Dibenzazepines |
|---|
| Direct Parent | Dibenzazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzazepine
- Alkyldiarylamine
- Tertiary aliphatic/aromatic amine
- Azepine
- Aryl chloride
- Aryl halide
- Benzenoid
- Tertiary amine
- Secondary amine
- Secondary aliphatic amine
- Azacycle
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-6090000000-9eed19a2ef45fc520c90 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-1069000000-86dede74ad37de04b372 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-6093000000-595de34abeb5c6686672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9240000000-ac7e79f4ac427320c754 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-f1e765f5a26c70ab77d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-1090000000-1ddc75254b4387dae329 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-2390000000-bb1e61bf6aeb82c055f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2009000000-c20e525f00993d151f84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-4095000000-6701457843879edd44f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0096-9040000000-fdf055a6b5c0574ca702 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-228c0f7f447a837fde13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0090000000-0c7b194f8eae2f796bbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2090000000-e19659de1a99730937fb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060947 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Norclomipramine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 124969 |
|---|
| PubChem Compound ID | 622606 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|