| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:42:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:33 UTC |
|---|
| Accession Number | CHEM022629 |
|---|
| Identification |
|---|
| Common Name | N-Acetylserotonin sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | N-Acetylserotonin sulfate is a metabolite of melatonin. Melatonin Listen/ˌmɛləˈtoʊnɪn/, also known chemically as N-acetyl-5-methoxytryptamine, is a naturally occurring compound found in animals, plants, and microbes. In animals, circulating levels of the hormone melatonin vary in a daily cycle, thereby allowing the entrainment of the circadian rhythms of several biological functions. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
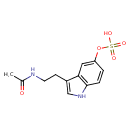
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Acetylserotonin sulfuric acid | Generator | | N-Acetylserotonin sulphate | Generator | | N-Acetylserotonin sulphuric acid | Generator | | N-{2-[5-(sulfooxy)-1H-indol-3-yl]ethyl}ethanimidate | Generator | | N-{2-[5-(sulphooxy)-1H-indol-3-yl]ethyl}ethanimidate | Generator | | N-{2-[5-(sulphooxy)-1H-indol-3-yl]ethyl}ethanimidic acid | Generator |
|
|---|
| Chemical Formula | C12H14N2O5S |
|---|
| Average Molecular Mass | 298.315 g/mol |
|---|
| Monoisotopic Mass | 298.062 g/mol |
|---|
| CAS Registry Number | 94840-68-3 |
|---|
| IUPAC Name | [3-(2-acetamidoethyl)-1H-indol-5-yl]oxidanesulfonic acid |
|---|
| Traditional Name | [3-(2-acetamidoethyl)-1H-indol-5-yl]oxidanesulfonic acid |
|---|
| SMILES | CC(=O)NCCC1=CNC2=C1C=C(OS(O)(=O)=O)C=C2 |
|---|
| InChI Identifier | InChI=1S/C12H14N2O5S/c1-8(15)13-5-4-9-7-14-12-3-2-10(6-11(9)12)19-20(16,17)18/h2-3,6-7,14H,4-5H2,1H3,(H,13,15)(H,16,17,18) |
|---|
| InChI Key | UCAJZNVFRVLULS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acetyl-2-arylethylamines. N-acetyl-2-arylethylamines are compounds containing an acetamide group that is N-linked to an arylethylamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | N-acetyl-2-arylethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acetyl-2-arylethylamine
- Arylsulfate
- 3-alkylindole
- Indole
- Indole or derivatives
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Substituted pyrrole
- Organic sulfuric acid or derivatives
- Pyrrole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Azacycle
- Organoheterocyclic compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000x-8490000000-fb2a8d9838eba9e8f519 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1090000000-c384683e0cc65d7314a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0avj-3490000000-b2943ecaebd008d67991 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9200000000-217540fe6d856301b699 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-2090000000-21e1e6e7de9d14a43e4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-7090000000-e25c7892de8289df567f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000y-9520000000-cffd73864c32a2b905a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4j-0090000000-5b5d8789be614885bce3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0390000000-63ec1c0dc30e0a7e7768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-2940000000-81ac1cc4246a93536e85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0005-0090000000-c51314b8733c513c675f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06r6-0960000000-d1c5f69b64fb5802f8a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1900000000-1bc1f50da74825a102fb | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060834 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 45479248 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|