| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:42:51 UTC |
|---|
| Update Date | 2016-11-09 01:17:33 UTC |
|---|
| Accession Number | CHEM022626 |
|---|
| Identification |
|---|
| Common Name | Hydroxynalidixic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Hydroxynalidixic acid is a metabolite of nalidixic acid. Nalidixic acid (tradenames Nevigramon, Neggram, Wintomylon and WIN 18,320) is the first of the synthetic quinolone antibiotics. In the technical sense, it is a naphthyridone, not a quinolone: its ring structure is a 1,8-naphthyridine nucleus that contains two nitrogen atoms, unlike quinoline, which has a single nitrogen atom. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
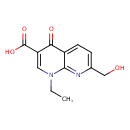
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Hydroxynalidixate | Generator | | 7-Hydroxymethylnalidixic acid | HMDB | | 7-(Hydroxymethyl)nalidixic acid | HMDB | | 7-Hydroxynalidixic acid, monosodium salt | HMDB | | 1-Ethyl-7-(hydroxymethyl)-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate | Generator |
|
|---|
| Chemical Formula | C12H12N2O4 |
|---|
| Average Molecular Mass | 248.235 g/mol |
|---|
| Monoisotopic Mass | 248.080 g/mol |
|---|
| CAS Registry Number | 3759-18-0 |
|---|
| IUPAC Name | 1-ethyl-7-(hydroxymethyl)-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid |
|---|
| Traditional Name | 1-ethyl-7-(hydroxymethyl)-4-oxo-1,8-naphthyridine-3-carboxylic acid |
|---|
| SMILES | CCN1C=C(C(O)=O)C(=O)C2=CC=C(CO)N=C12 |
|---|
| InChI Identifier | InChI=1S/C12H12N2O4/c1-2-14-5-9(12(17)18)10(16)8-4-3-7(6-15)13-11(8)14/h3-5,15H,2,6H2,1H3,(H,17,18) |
|---|
| InChI Key | FRJDYPGZHOEEKU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthyridine carboxylic acids and derivatives. Naphthyridine carboxylic acids and derivatives are compounds containing a naphthyridine moiety, where one of the ring atoms bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazanaphthalenes |
|---|
| Sub Class | Naphthyridines |
|---|
| Direct Parent | Naphthyridine carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthyridine carboxylic acid
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Pyridine
- Vinylogous amide
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Alcohol
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Aromatic alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lr-0190000000-28f2f7f2988c55589152 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fi0-4019000000-a10444a5136d59af29a7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0090000000-365c68eb53750e3a3946 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qi-0390000000-78daaca12050e9e8321c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0930000000-b93c37cc37c43bf9d863 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udj-0290000000-8eeeb403c32c462eee53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fmr-0920000000-b2124cd08c9905812582 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fr-0900000000-122cc11f732b6c39a720 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-c5fb311b8234402b13ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-0bbc16def421dfe3548d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0590000000-24f198534a5ff724bb79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ftb-0490000000-9b486fbafeb0162a41d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-bf0d9092c5b01be34aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0900000000-b7020ed63c54f3151501 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060826 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 160716 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|