| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:42:10 UTC |
|---|
| Update Date | 2016-11-09 01:17:32 UTC |
|---|
| Accession Number | CHEM022615 |
|---|
| Identification |
|---|
| Common Name | 3-O-Methyl-a-methyldopa |
|---|
| Class | Small Molecule |
|---|
| Description | A L-tyrosine derivative that is the 3-methoxy derivative of L-dopa. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
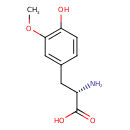
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-3-(4-Hydroxy-3-methoxyphenyl)-alanine | ChEBI | | 3-Methoxytyrosine | HMDB | | 3-Methoxytyrosine, (D-tyr)-isomer | HMDB | | 3-Methoxytyrosine, (L-tyr)-isomer, alpha-(14)C-labeled | HMDB | | 3-Methoxytyrosine, (DL-tyr)-isomer | HMDB | | 3-Methoxytyrosine, (L-tyr)-isomer | HMDB | | L-3-Methoxytyrosine | HMDB | | 3-Methoxydopa | MeSH | | 3-O-Methyl-dopa | MeSH | | 3-O-MethylDOPA | MeSH | | 3-OMD CPD | MeSH |
|
|---|
| Chemical Formula | C10H13NO4 |
|---|
| Average Molecular Mass | 211.215 g/mol |
|---|
| Monoisotopic Mass | 211.084 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S)-2-amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid |
|---|
| Traditional Name | 3-methoxytyrosine |
|---|
| SMILES | COC1=C(O)C=CC(C[C@H](N)C(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C10H13NO4/c1-15-9-5-6(2-3-8(9)12)4-7(11)10(13)14/h2-3,5,7,12H,4,11H2,1H3,(H,13,14)/t7-/m0/s1 |
|---|
| InChI Key | PFDUUKDQEHURQC-ZETCQYMHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tyrosine and derivatives. Tyrosine and derivatives are compounds containing tyrosine or a derivative thereof resulting from reaction of tyrosine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Tyrosine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tyrosine or derivatives
- Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- Methoxyphenol
- L-alpha-amino acid
- Phenoxy compound
- Phenol ether
- Anisole
- Methoxybenzene
- Phenol
- Alkyl aryl ether
- Aralkylamine
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Amino acid
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014u-3900000000-77ff6cac3b58ca81edf2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00y3-9262000000-310ac3872da5a80ae833 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02t9-0930000000-c2ec893f0b2d690b8149 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-a91930d8f8538ef1574f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3900000000-1cea4dc97d1eb674a768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0290000000-37322740518e056012f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1950000000-7d97cb07b9e8e3079548 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-7900000000-e99315a95aa2bdd3c30c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03xr-0980000000-e506ae114e636e7fe4a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1910000000-edbdead984fe371e39de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-4900000000-7cc4de1760e6f692036f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-78e52f9c7af96bfee33a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-6900000000-70c616cb8fba2824ccdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fkc-9500000000-4e3895853fa75df2bcb1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060747 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | 3YM |
|---|
| Wikipedia Link | 3-O-Methyldopa |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 82913 |
|---|
| PubChem Compound ID | 9307 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|