| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:41:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:32 UTC |
|---|
| Accession Number | CHEM022596 |
|---|
| Identification |
|---|
| Common Name | Nor-ketotifen |
|---|
| Class | Small Molecule |
|---|
| Description | Nor-ketotifen is a metabolite of ketotifen. Ketotifen is a second-generation H1-antihistamine and mast cell stabilizer. It is most commonly sold in as a salt of fumaric acid, ketotifen fumarate, and is available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis, or the itchy red eyes caused by allergies. In its oral form, it is used to prevent asthma attacks. Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
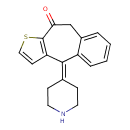
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Norketotifen | HMDB |
|
|---|
| Chemical Formula | C18H17NOS |
|---|
| Average Molecular Mass | 295.399 g/mol |
|---|
| Monoisotopic Mass | 295.103 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-(piperidin-4-ylidene)-6-thiatricyclo[8.4.0.0³,⁷]tetradeca-1(14),3(7),4,10,12-pentaen-8-one |
|---|
| Traditional Name | 2-(piperidin-4-ylidene)-6-thiatricyclo[8.4.0.0³,⁷]tetradeca-1(14),3(7),4,10,12-pentaen-8-one |
|---|
| SMILES | O=C1CC2=CC=CC=C2C(C2=C1SC=C2)=C1CCNCC1 |
|---|
| InChI Identifier | InChI=1S/C18H17NOS/c20-16-11-13-3-1-2-4-14(13)17(12-5-8-19-9-6-12)15-7-10-21-18(15)16/h1-4,7,10,19H,5-6,8-9,11H2 |
|---|
| InChI Key | IYSYPCSSDZBWHN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cycloheptathiophenes. These are polycyclic compounds containing a thiophene ring fused to a 7 member carbocyclic moiety. Thiophene is 5-membered ring consisting of four carbon atoms and one sulfur atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Cycloheptathiophenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Cycloheptathiophenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cycloheptathiophene
- Aryl alkyl ketone
- Aryl ketone
- Piperidine
- Benzenoid
- Heteroaromatic compound
- Thiophene
- Ketone
- Secondary aliphatic amine
- Secondary amine
- Azacycle
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0i29-1390000000-346c9802ccc53d5dacd6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1090000000-e2cb58a3df61c5309a87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000t-4290000000-0e1ac68ba3732addc16b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9450000000-bf76bf6891a2589b22b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-b9973c819fa3f421efdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-375ad310ec4cb984a93c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9030000000-4d24ba2224f3141456c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-8462ed387db681137e5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-50e52666a71cd4b27b2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fbi-1090000000-023e2cf56c1325a0efdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-40e2e7fc956768de3dcd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-843235c69b8c710c5f73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002u-0090000000-8cdf8b891811c1557d88 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060646 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8079890 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9904236 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|