| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:40:06 UTC |
|---|
| Update Date | 2016-11-09 01:17:32 UTC |
|---|
| Accession Number | CHEM022578 |
|---|
| Identification |
|---|
| Common Name | Mycophenolic acid O-acyl-glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | A carboxylic ester resulting from the formal condensation of the carboxylic acid group of mycophenolic acid with the anomeric hydroxy group of beta-D-glucuronic acid. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
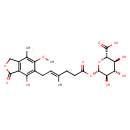
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| AcMPAG | ChEBI | | Mycophenolic acid acyl glucuronide | ChEBI | | Mycophenolic acid acyl-glucuronide | ChEBI | | Mycophenolic acid-O-acyl-beta-D-glucopyranuronoside | ChEBI | | Mycophenolate acyl glucuronide | Generator | | Mycophenolate acyl-glucuronide | Generator | | Mycophenolate-O-acyl-b-D-glucopyranuronoside | Generator | | Mycophenolate-O-acyl-beta-D-glucopyranuronoside | Generator | | Mycophenolate-O-acyl-β-D-glucopyranuronoside | Generator | | Mycophenolic acid-O-acyl-b-D-glucopyranuronoside | Generator | | Mycophenolic acid-O-acyl-β-D-glucopyranuronoside | Generator | | Mycophenolate O-acyl-glucuronide | Generator |
|
|---|
| Chemical Formula | C23H28O12 |
|---|
| Average Molecular Mass | 496.461 g/mol |
|---|
| Monoisotopic Mass | 496.158 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[(4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoyl]oxy}oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[(4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoyl]oxy}oxane-2-carboxylic acid |
|---|
| SMILES | COC1=C(C)C2=C(C(=O)OC2)C(O)=C1C\C=C(/C)CCC(=O)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H28O12/c1-9(4-6-11-15(25)14-12(8-33-22(14)31)10(2)19(11)32-3)5-7-13(24)34-23-18(28)16(26)17(27)20(35-23)21(29)30/h4,16-18,20,23,25-28H,5-8H2,1-3H3,(H,29,30)/b9-4+/t16-,17-,18+,20-,23+/m0/s1 |
|---|
| InChI Key | QBMSTEZXAMABFF-UEARNRKISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glucuronides. These are glucuronides in which the aglycone is linked to the carbohydrate unit through an O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glucuronides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-o-glucuronide
- O-glucuronide
- Isobenzofuranone
- Phthalide
- Isocoumaran
- Tricarboxylic acid or derivatives
- Anisole
- Alkyl aryl ether
- Beta-hydroxy acid
- Fatty acid ester
- Fatty acyl
- Hydroxy acid
- Benzenoid
- Monosaccharide
- Pyran
- Oxane
- Vinylogous acid
- Secondary alcohol
- Lactone
- Carboxylic acid ester
- Acetal
- Organoheterocyclic compound
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Polyol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056r-9132400000-8d1d445b5c92387b8fdd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-9807007000-7f6173b63131c0199045 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Mycophenolic acid O-acyl-glucuronide,3TBDMS,#6" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1229400000-a00fd0b14652a8a073e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v00-3927200000-b03a15a97c3652e63eb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0miy-9725000000-7d44f74d3818db669d41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0001900000-3a9bf5cebae751792dee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ktb-2054900000-3d6868acf21096f78353 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-1192200000-4eb5e92df9f368cdbdd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fi1-0539500000-bfc6fff89aeca9bc0aeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kk9-1898200000-2b4abbcf7a3d676e09ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-3951000000-bf27b3a68a384eb61846 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0032900000-688e9215c56e8c6bc792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0091000000-e2000425e22fefbd07ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014j-0091100000-9d561f09ce0219cf3eaa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060491 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64689 |
|---|
| PubChem Compound ID | 10028772 |
|---|
| Kegg Compound ID | C20383 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|