| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:37:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:31 UTC |
|---|
| Accession Number | CHEM022510 |
|---|
| Identification |
|---|
| Common Name | Nicotine glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
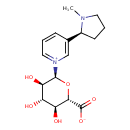
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-1-alpha-D-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)pyridinium inner salt | ChEBI | | 3,4,5-Trihydroxy-6-[5-(1-methylpyrrolidin-2-yl)pyridin-1-yl]-oxane-2-carboxylate | ChEBI | | Nicotine N-glucuronide | ChEBI | | (S)-1-a-D-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)pyridinium inner salt | Generator | | (S)-1-Α-D-glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)pyridinium inner salt | Generator | | 3,4,5-Trihydroxy-6-[5-(1-methylpyrrolidin-2-yl)pyridin-1-yl]-oxane-2-carboxylic acid | Generator | | (S)-1-alpha-D-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)-pyridinium inner salt | HMDB | | (S)-1-alpha-delta-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)-pyridinium inner salt | HMDB | | (S)-1-alpha-delta-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)pyridinium inner salt | HMDB | | 1-alpha-D-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)-(S)-pyridinium | HMDB | | 1-alpha-D-Glucopyranuronosyl-3-[(2S)-1-methyl-2-pyrrolidinyl]-pyridinium | HMDB | | 1-alpha-delta-Glucopyranuronosyl-3-(1-methyl-2-pyrrolidinyl)-(S)-pyridinium | HMDB | | 1-alpha-delta-Glucopyranuronosyl-3-[(2S)-1-methyl-2-pyrrolidinyl]-pyridinium | HMDB | | Nicotine-glucuronide | HMDB |
|
|---|
| Chemical Formula | C16H22N2O6 |
|---|
| Average Molecular Mass | 338.356 g/mol |
|---|
| Monoisotopic Mass | 338.148 g/mol |
|---|
| CAS Registry Number | 152306-59-7 |
|---|
| IUPAC Name | 1-[(2S,3R,4S,5S,6S)-6-carboxylato-3,4,5-trihydroxyoxan-2-yl]-3-[(2S)-1-methylpyrrolidin-2-yl]-1λ⁵-pyridin-1-ylium |
|---|
| Traditional Name | nicotine glucuronide |
|---|
| SMILES | CN1CCC[C@H]1C1=C[N+](=CC=C1)[C@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C16H22N2O6/c1-17-6-3-5-10(17)9-4-2-7-18(8-9)15-13(21)11(19)12(20)14(24-15)16(22)23/h2,4,7-8,10-15,19-21H,3,5-6H2,1H3/t10-,11-,12-,13+,14-,15-/m0/s1 |
|---|
| InChI Key | SAWAIULJDYFLPD-SOAFEQHCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as purine ribonucleoside triphosphates. These are purine ribobucleotides with a triphosphate group linked to the ribose moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
| Direct Parent | Purine ribonucleoside triphosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine ribonucleoside triphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Hydroxypyrimidine
- Alkyl phosphate
- Pyrimidine
- Monosaccharide
- Phosphoric acid ester
- N-substituted imidazole
- Organic phosphoric acid derivative
- Heteroaromatic compound
- Azole
- Imidazole
- Tetrahydrofuran
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-9363000000-498032dac1d25a048f1c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-000l-6695530000-5f3cb4632382491d1884 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-c0a6517967854466764f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-3209000000-9c96a94c455824fbcb27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xu-6911000000-358133c8eb23ea49be3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-470460c450e2ce5dc00f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-5594000000-c8f1d538ca13bdab946d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9200000000-83503a4041514150d674 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0209000000-45aaad3735689e97aa1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03yr-0905000000-ca062e765a6a892950c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-3910000000-d6d53f13a1744fd96f8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0229000000-8425edbf57e8743a529b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06ri-5977000000-71e200566908d48e305d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-8941000000-0a955908878509d2a872 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001272 |
|---|
| FooDB ID | FDB022526 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6127 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2299987 |
|---|
| ChEBI ID | 63860 |
|---|
| PubChem Compound ID | 3035848 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Heavner DL, Richardson JD, Morgan WT, Ogden MW: Validation and application of a method for the determination of nicotine and five major metabolites in smokers' urine by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2005 May;19(4):312-28. | | 2. Tulunay OE, Hecht SS, Carmella SG, Zhang Y, Lemmonds C, Murphy S, Hatsukami DK: Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1283-6. | | 3. Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK: Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999 Feb 1;59(3):590-6. | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=11201307 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=11846386 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=15265511 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=21497036 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=7902257 |
|
|---|