| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:37:31 UTC |
|---|
| Update Date | 2016-11-09 01:17:31 UTC |
|---|
| Accession Number | CHEM022496 |
|---|
| Identification |
|---|
| Common Name | Test Metabolite |
|---|
| Class | Small Molecule |
|---|
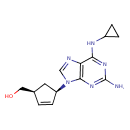
| Description | Abacavir (ABC) is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS. Chemically, it is a synthetic carbocyclic nucleoside and is the enantiomer with 1S, 4R absolute configuration on the cyclopentene ring. In vivo, Abacavir dissociates to its free base, abacavir. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| ABC | ChEBI | | {(1S-cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol | ChEBI | | (1S,4R)-4-(2-Amino-6-(cyclopropylamino)-9H-purin-9-yl)-2-cyclopentene-1-methanol | HMDB | | Abacavir succinate | HMDB | | Ziagen | HMDB | | Abacavir sulfate | HMDB |
|
|---|
| Chemical Formula | C14H18N6O |
|---|
| Average Molecular Mass | 286.332 g/mol |
|---|
| Monoisotopic Mass | 286.154 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl]methanol |
|---|
| Traditional Name | abacavir |
|---|
| SMILES | NC1=NC2=C(N=CN2[C@@H]2C[C@H](CO)C=C2)C(NC2CC2)=N1 |
|---|
| InChI Identifier | InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1 |
|---|
| InChI Key | MCGSCOLBFJQGHM-SCZZXKLOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-substituted cyclopentyl purine nucleosides. These are nucleoside analogues with a structure that consists of a cyclobutane that is substituted a the 1-position with a hydroxyl group and at the 3-position with either a purine base. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Nucleoside and nucleotide analogues |
|---|
| Sub Class | Cyclopentyl nucleosides |
|---|
| Direct Parent | 1,3-substituted cyclopentyl purine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-substituted cyclopentyl purine nucleoside
- 6-alkylaminopurine
- 6-aminopurine
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Secondary aliphatic/aromatic amine
- N-substituted imidazole
- Pyrimidine
- Imidolactam
- Heteroaromatic compound
- Azole
- Imidazole
- Secondary amine
- Organoheterocyclic compound
- Azacycle
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Primary alcohol
- Primary amine
- Organic oxygen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-4290000000-ff09f3896a50abd46e5c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0005-9257000000-21b4bbc1ab23c07507d6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0090000000-0946b359f044edc1eaa7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2190000000-64675b2273332e37f39a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9410000000-d05153b2e67c168956b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-2944c593361dbd76b614 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-2390000000-06a2fd880fff3ed2e268 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9630000000-7c461fd855fa0cc2d7b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-0790000000-368d898ea37a7e409e6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-fcb41525305a5be74f3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0920000000-ce3e8f7641b332656356 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e97d53ff96d49e16f45a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0190000000-992655fbf9c97b5fcce1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0930000000-f24bb2b6a90e0a5559a1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01048 |
|---|
| HMDB ID | HMDB0015182 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Abacavir |
|---|
| Chemspider ID | 390063 |
|---|
| ChEBI ID | 421707 |
|---|
| PubChem Compound ID | 441300 |
|---|
| Kegg Compound ID | C07624 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|