| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:35:03 UTC |
|---|
| Update Date | 2016-11-09 01:17:30 UTC |
|---|
| Accession Number | CHEM022441 |
|---|
| Identification |
|---|
| Common Name | 5-alpha-Dihydrotestosterone glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
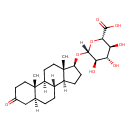
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5alpha,17beta)-3-Oxoandrostan-17-yl beta-D-glucopyranosiduronic acid | ChEBI | | 17beta-Hydroxy-5alpha-androstan-3-one glucuronide | ChEBI | | 5alpha-Dihydrotestosterone 17-(beta-D-glucuronide) | ChEBI | | 5alpha-Dihydrotestosterone 17-beta-glucuronide | ChEBI | | 5alpha-Dihydrotestosterone 17-O-(beta-D-glucuronic acid) | ChEBI | | 5alpha-Dihydrotestosterone glucuronide | ChEBI | | 5alpha-Dihydrotestosterone-17g | ChEBI | | Dihydrotestosterone 17-glucuronide | ChEBI | | Dihydrotestosterone glucuronide | ChEBI | | Stanolone 17-glucuronide | ChEBI | | Stanolone glucuronate | ChEBI | | (5a,17b)-3-Oxoandrostan-17-yl b-D-glucopyranosiduronate | Generator | | (5a,17b)-3-Oxoandrostan-17-yl b-D-glucopyranosiduronic acid | Generator | | (5alpha,17beta)-3-Oxoandrostan-17-yl beta-D-glucopyranosiduronate | Generator | | (5Α,17β)-3-oxoandrostan-17-yl β-D-glucopyranosiduronate | Generator | | (5Α,17β)-3-oxoandrostan-17-yl β-D-glucopyranosiduronic acid | Generator | | 17b-Hydroxy-5a-androstan-3-one glucuronide | Generator | | 17Β-hydroxy-5α-androstan-3-one glucuronide | Generator | | 5a-Dihydrotestosterone 17-(b-D-glucuronide) | Generator | | 5Α-dihydrotestosterone 17-(β-D-glucuronide) | Generator | | 5a-Dihydrotestosterone 17-b-glucuronide | Generator | | 5Α-dihydrotestosterone 17-β-glucuronide | Generator | | 5a-Dihydrotestosterone 17-O-(b-D-glucuronate) | Generator | | 5a-Dihydrotestosterone 17-O-(b-D-glucuronic acid) | Generator | | 5alpha-Dihydrotestosterone 17-O-(beta-D-glucuronate) | Generator | | 5Α-dihydrotestosterone 17-O-(β-D-glucuronate) | Generator | | 5Α-dihydrotestosterone 17-O-(β-D-glucuronic acid) | Generator | | 5a-Dihydrotestosterone glucuronide | Generator | | 5Α-dihydrotestosterone glucuronide | Generator | | 5a-Dihydrotestosterone-17g | Generator | | 5Α-dihydrotestosterone-17g | Generator | | Stanolone glucuronic acid | Generator | | 5-a-Dihydrotestosterone glucuronide | Generator | | 5-Α-dihydrotestosterone glucuronide | Generator | | Dihydrotestosterone diglucuronide | HMDB | | Dihydrotestosterone glucuronide, (5beta,17beta)-isomer | HMDB |
|
|---|
| Chemical Formula | C25H38O8 |
|---|
| Average Molecular Mass | 466.564 g/mol |
|---|
| Monoisotopic Mass | 466.257 g/mol |
|---|
| CAS Registry Number | 42037-24-1 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(1S,2S,7S,10R,11S,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-6-{[(1S,2S,7S,10R,11S,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| SMILES | [H][C@@]12CC[C@H](O[C@]3([H])O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C25H38O8/c1-24-9-7-13(26)11-12(24)3-4-14-15-5-6-17(25(15,2)10-8-16(14)24)32-23-20(29)18(27)19(28)21(33-23)22(30)31/h12,14-21,23,27-29H,3-11H2,1-2H3,(H,30,31)/t12-,14-,15-,16-,17-,18-,19-,20+,21-,23+,24-,25-/m0/s1 |
|---|
| InChI Key | CLQMBSSRTBUNDV-CPKOJWPQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Androgen-skeleton
- Androstane-skeleton
- 3-oxosteroid
- 3-oxo-5-alpha-steroid
- Oxosteroid
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- O-glycosyl compound
- Glycosyl compound
- Beta-hydroxy acid
- Hydroxy acid
- Oxane
- Monosaccharide
- Pyran
- Cyclic ketone
- Secondary alcohol
- Ketone
- Polyol
- Acetal
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007a-6264900000-caa236f8fcb97291d23b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-1261029000-94f9c3d5903740431670 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r7-0190600000-74d7352cda2362ddb906 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0290000000-8dcf4c902df6743a8f9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-0490000000-278db46d2af73936b3be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01bi-1260900000-3c796e68ff231c8976b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1290200000-f56012a4058dcff174ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-4190000000-14bb869866e58062e6fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-8ccc8d81a1705cd514f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ara-9421500000-426185885a7fcd23f188 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9103100000-942b47d6aa0fb6b5c1ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ba-0150900000-8a78977927165645dce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vk-0954600000-159af7866ca65a3c8336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-1911000000-5865b00a94ace6eedd9f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006203 |
|---|
| FooDB ID | FDB023835 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 2423365 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24850129 |
|---|
| ChEBI ID | 89417 |
|---|
| PubChem Compound ID | 44263365 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Choi MH, Kim JN, Chung BC: Rapid HPLC-electrospray tandem mass spectrometric assay for urinary testosterone and dihydrotestosterone glucuronides from patients with benign prostate hyperplasia. Clin Chem. 2003 Feb;49(2):322-5. | | 2. Murai T, Samata N, Iwabuchi H, Ikeda T: Human UDP-glucuronosyltransferase, UGT1A8, glucuronidates dihydrotestosterone to a monoglucuronide and further to a structurally novel diglucuronide. Drug Metab Dispos. 2006 Jul;34(7):1102-8. Epub 2006 Apr 4. | | 3. Prevost J, Brochu M, Belanger A, Lambert R: Conjugated and unconjugated C-21, C-19 and C-18 steroid concentrations in human follicular fluid from hyperstimulated follicles. Gynecol Endocrinol. 1987 Dec;1(4):331-8. | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=10624837 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=12560362 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=16595710 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=263350 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=2747415 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=3140586 |
|
|---|