| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:32:18 UTC |
|---|
| Update Date | 2016-11-09 01:17:30 UTC |
|---|
| Accession Number | CHEM022424 |
|---|
| Identification |
|---|
| Common Name | 2-Amino-4-hydroxy-6-pteridinecarboxylic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Amino-4-hydroxy-6-pteridinecarboxylic acid is found in fishes. 2-Amino-4-hydroxy-6-pteridinecarboxylic acid is isolated from various biol. sources including fish and soybeans. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
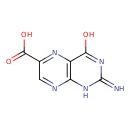
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-4-hydroxy-6-pteridinecarboxylate | Generator | | 2-amino-1,4-dihydro-4-oxo-6-Pteridinecarboxylic acid | HMDB | | 2-amino-1,4-dihydro-4-Oxopteridine-6-carboxylic acid | HMDB | | 2-amino-4(3H)-Pteridinone-6-carboxylic acid | HMDB | | 2-amino-4-Hydroxypteridine-6-carboxylic acid | HMDB | | 2-amino-4-Hydroxypteridine-6-carboxylicacid | HMDB | | 2-amino-4-oxo-3,4-dihydro-6-Pteridinecarboxylic acid | HMDB | | HHS | HMDB | | Pterin-6-carboxylic acid | HMDB | | Pterine-6-carboxylic acid | HMDB | | Ranachrome 5 | HMDB | | 4-Hydroxy-2-imino-1,2-dihydropteridine-6-carboxylate | Generator |
|
|---|
| Chemical Formula | C7H5N5O3 |
|---|
| Average Molecular Mass | 207.146 g/mol |
|---|
| Monoisotopic Mass | 207.039 g/mol |
|---|
| CAS Registry Number | 948-60-7 |
|---|
| IUPAC Name | 2-amino-4-oxo-3,4-dihydropteridine-6-carboxylic acid |
|---|
| Traditional Name | 2-amino-4-oxo-3H-pteridine-6-carboxylic acid |
|---|
| SMILES | NC1=NC2=NC=C(N=C2C(=O)N1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H5N5O3/c8-7-11-4-3(5(13)12-7)10-2(1-9-4)6(14)15/h1H,(H,14,15)(H3,8,9,11,12,13) |
|---|
| InChI Key | QABAUCFGPWONOG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pterin carboxylates. These are heterocyclic aromatic compounds containing a pterin moiety, in which one ring is substituted by one or more carboxylic acid groups. Pterin is a heterocyclic compound composed of a pteridine ring system (made up of a pyrazine fused to a pyrimidine), with a keto group and an amino group at the 2- and 4- positions, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Pterin carboxylates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pterin-6-carboxylate
- Pyrazine carboxylic acid
- Pyrazine carboxylic acid or derivatives
- Hydroxypyrimidine
- Pyrimidine
- Pyrazine
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-3920000000-3d76f8b1533d1ce1c488 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9680000000-8da2aac44785ee38b58d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0980000000-b3f5864ba11d3311461a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06r6-0920000000-e3fa4c4cac02e924df9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dm-1900000000-fd438b3ccb48b7d6a3ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0590000000-24af2fda53c2bbf01add | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08g0-1930000000-3e2f8348c2ca92ce8d2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9600000000-968da7fac93560009529 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-99937f8026369fb0e183 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0910000000-4b273b17654da5dfc29b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-7950000000-9cdfb1ff9c53dbb1feb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-81d7d4c124979da045f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0910000000-ec91dfa8dbc360f55773 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ls-9710000000-b1367905315aee6f1e37 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033136 |
|---|
| FooDB ID | FDB011137 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 63542 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 70361 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|