| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:32:14 UTC |
|---|
| Update Date | 2016-11-09 01:17:30 UTC |
|---|
| Accession Number | CHEM022422 |
|---|
| Identification |
|---|
| Common Name | 1,2-Dihydro-1,1,6-trimethylnaphthalene |
|---|
| Class | Small Molecule |
|---|
| Description | 1,2-Dihydro-1,1,6-trimethylnaphthalene is found in alcoholic beverages. 1,2-Dihydro-1,1,6-trimethylnaphthalene is isolated from strawberry oil, peaches, tobacco and wines. 1,2-Dihydro-1,1,6-trimethylnaphthalene is a component of wine off-flavour on ageing. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
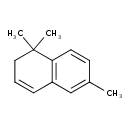
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,1,6-Trimethyl-1,2-dihydro-naphthalene | HMDB | | 1,1,6-Trimethyl-1,2-dihydronaphthalene | HMDB | | 1,1,6-Trimethyl-2H-naphthalene | HMDB | | 1,2-dihydro-1,5,8-Trimethylnaphthalene | HMDB | | 1,5,8-Trimethyl-1,2-dihydronaphthalene | HMDB | | 3,4-Dehydroionene | HMDB | | dehydro-AR-ionene | HMDB | | Napthalene,1,2,diydro-1,1,6-trimethyl | HMDB | | TDN | HMDB | | TDN Compound | MeSH, HMDB |
|
|---|
| Chemical Formula | C13H16 |
|---|
| Average Molecular Mass | 172.266 g/mol |
|---|
| Monoisotopic Mass | 172.125 g/mol |
|---|
| CAS Registry Number | 30364-38-6 |
|---|
| IUPAC Name | 1,1,6-trimethyl-1,2-dihydronaphthalene |
|---|
| Traditional Name | 1,1,6-trimethyl-2H-naphthalene |
|---|
| SMILES | CC1=CC2=C(C=C1)C(C)(C)CC=C2 |
|---|
| InChI Identifier | InChI=1S/C13H16/c1-10-6-7-12-11(9-10)5-4-8-13(12,2)3/h4-7,9H,8H2,1-3H3 |
|---|
| InChI Key | RTUMCNDCAVLXEP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Aromatic hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a59-0900000000-67eeac320b4e4ac54af9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-f86aa5ce6175c1838be4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1900000000-5915302a8f7fc1b77faa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-067i-7900000000-55efd4a444b145b084c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-7b3cff0cd89bae4893cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-7b3cff0cd89bae4893cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-0900000000-92fb9615dd967599c0b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-49b5e780d25922a8ac44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-a7c73ae55b5eaf7b19f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-4900000000-b0c33775c93cc9b12ab3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-931fce372207769999ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-931fce372207769999ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0900000000-d92a1777757f13ab332a | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040284 |
|---|
| FooDB ID | FDB020005 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055601 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 108567 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 121677 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01576 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|